Understanding the relative strengths and weaknesses of different package materials is critical in selecting the best medical packaging system for a particular device.
January 1, 2000
Originally Published January 2000
Selecting medical packaging materials requires careful consideration of the functional requirements of the package and method of packaging. The critical nature of sterility maintenance requires a thorough understanding of the design and function of the medical device and the machinery being used to package it. For packaging systems employing a heat seal, the user is faced with several options in medical packaging materials. This article will review seal/peel systems based on heat seal coatings and peelable resins, and attempt to assist device manufacturers in choosing the proper system for their packaging application. Topics covered include the medical packaging equipment, method of medical packaging sterilization, desired seal/peel properties, overall medical package cost, and new medical packaging material offerings.
MEDICAL PACKAGING EQUIPMENT
The characteristics of the medical packaging material chosen should be compatible with the type of packaging equipment being employed. Different types of equipment place different demands on the package. Generally, heat seal machinery falls into one of two categories: intermittent or continuous sealing. Examples of intermittent sealing equipment, which frequently employs a platen in the creation of the seal, include form-fill-seal (FFS) units, tray sealers, and pouch machines. These machines generally apply heat through the top web and place minimal stress on the package because of their longer dwell times. In intermittent or platen systems, the packaging typically remains stationary throughout the heat sealing cycle. Intermittent heat sealing equipment can operate with either flexible or rigid substrates.
Continuous sealing equipment—such as, for example, a rotary four-side sealer—presents a larger challenge due to extremely short dwell time during the heat sealing process—as little as one-tenth or less of the dwell time available with platen systems. Because of this, continuous sealing equipment places more stress on a medical package, challenging the package integrity very soon after the formation of the package seals. The ability of a seal to resist opening immediately after being formed is called hot tack, and it is this property that is often critically important for packaging materials used on rotary machines. Additionally, short residence times on the heat sealing sections of rotary equipment mean that sealant materials have to activate quickly. To assist in this process, some manufacturers' machines apply heat through both the top and bottom webs.
Packaging Machine | 2-D or 3-D | Seal Time | Seal Conditions | Seal Stress |
Tray sealer | 3-D | Long | Low stress | Minimum unless lid sticks to sealer |
Transverse FFS | 3-D | Medium | Good pressure | Machine index stress and cutting stress |
Platten die | 3-D | Medium | Ideal | Minimum if low-profile device |
Rotary four-side | 2-D | Minimal | High-temp die | Worst hot-tack stress—varies around permimeter |
Table I. Packaging machine sealing characteristics.
Heat seal–coated (HSC) materials can be formulated to have excellent hot-tack properties, and can therefore form seals under a wide array of sealing conditions, from rotary 2-D sealing operations to slower 3-D tray or lid applications. This enables HSC materials to perform well on both continuous- and platen-type equipment. HSC materials are versatile and will adhere to a variety of rigid and flexible substrates, in contrast to most peel-able films, which form seals only to similar polyethylene-based materials (such as flexible films). Peelable films typically require longer dwell times for sealing, and thus are well suited for platen-type equipment. Table I summarizes the different heat sealing characteristics found in various medical packaging machinery.
HOW STERILIZATION
There are a number of methods available for terminal sterilization of a medical device, and new methods are introduced to the marketplace on a fairly regular basis. Three of the most common methods employed by device manufacturers are ethylene oxide (EtO) gas, high-energy radiation, and autoclaving. Other sterilization methods include chemiclaving, dry heat, and plasma. Each requires that careful thought be given to the specific materials used in the medical packaging under consideration. The discussion in this article will generally be restricted to EtO and radiation sterilization, though many of the conclusions are not limited to these two techniques.
In general, sterilization methods using a gas (such as EtO) require a permeable material, as the gas must pass in and out of the medical package to sterilize the contents. Both HSC materials and resin-based peel systems can be used with EtO, provided some portion of the medical package exhibits sufficient porosity to admit the sterilizing gas—as would be the case with paper or Tyvek, for example. The process of cycling gases in and out of the package can itself put stress on the package seals, and is yet another area in which careful choices need to be made to ensure that materials and seals are capable of maintaining medical package integrity.
Peelable films and HSC materials are also well suited for radiation sterilization. In the medical industry, the two widely employed types of radiation are gamma (cobalt 60 particles) and electron beam (ß particles). Electron beam is typically used for less-dense materials due to the shallower penetration depth of the lower-energy ß particles. Radiation sterilization does not require porous medical packaging materials, and is frequently the sterilization method chosen when a barrier to gas penetration is a requirement of the medical device. However, radiation is frequently employed with permeable package types if the medical packaging material properties will not be compromised during treatment.
Material | Irradiation | Ethylene Oxide | Autoclave | Dry Heat |
Polyethylene | Satisfactory | Satisfactory | Not suitable | Not suitable |
PVC film | Discolors | Satisfactory | Not suitable | Not suitable |
Ionomer resin | Satisfactory | Satisfactory | Not suitable | Not suitable |
Polyamides | OK if laminated | Satisfactory | Satisfactory | Satisfactory |
Polyester | Satisfactory | Satisfactory | Satisfactory | Satisfactory |
Polypropylene | Embrittles | Satisfactory | Satisfactory | Satisfactory |
Polycarbonate | Satisfactory | Satisfactory | Satisfactory | Satisfactory |
Polystyrene | Satisfactory | Satisfactory | Not suitable | Marginal |
Table II. Polymer sterilization compatability.
Steam autoclave sterilization requires higher-molecular-weight, thermally stable medical packaging materials such as polypropylene or polycarbonate. Peelable films compatible with such sterilization methods are not widely available at present. As a result, heat seal coatings dominate this application. Table II provides an illustration of various polymers and their suitability for different forms of sterilization.
PEEL/SEAL MEDICAL PACKAGING TECHNOLOGY
For the purpose of this article, peelability will be defined as the ability to separate two materials in the course of opening a package without compromising the integrity of either of the two. In medical packaging, a peelable system provides a controlled, reliable, aseptic means of opening a package and presenting a device. The sealant layer of one or both webs is responsible for bonding the two materials together, which is accomplished via the application of heat. Typically, the larger the temperature range over which a seal can be formed, the better. A wide sealing window allows for greater versatility, easing processing concerns and increasing the reliability of the medical package.
The force required to pull a seal apart is called its seal strength. Seal strength in a peelable system is controlled by the composition of either the heat seal coating or the sealant layer. Typical medical packages have a seal strength of 1–3 lb per in. of seal width, as measured via a standard test such as ASTM F88-94.
Peelable films are generally based on polybutylene-polyolefin technology first pioneered by Shell in the mid-1970s. The incompatibility of the two polymers inhibits the sealant layer from forming a complete bond by reducing the number of available bonding sites. These peelable systems provide seal transfer by internal cohesive splitting between the polyethylene and polybutylene layers because of poor interfacial adhesion, which reduces internal bond strength. This is in contrast to HSC materials, which undergo the cohesive failure that occurs when the internal strength of the adhesive is less than the strength of the bonds between the adhesive and sealed materials.
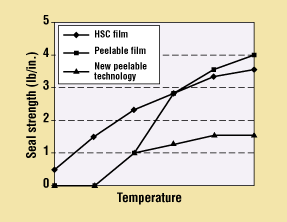 Figure 1. Seal-strength comparison of peelable films versus heat-seal-coated film.
Figure 1. Seal-strength comparison of peelable films versus heat-seal-coated film.
Peelable films are generally limited to similar-type materials that are primarily polyethylene (PE) based, and tend to have a narrower sealing window and/or a steeper peel-strength slope compared with HSC materials. However, new peelable technologies are being introduced that can provide increased sealing windows with smaller variations in peel strength over their useful range. Figure 1 illustrates the typical seal-strength curves of the various types of material when sealed to polyethylene film. These new peelable resin systems are being developed to seal to a wide variety of materials, including but not limited to PETG, HIPS, and PVC.
HOT TACK MEDICAL PACKAGING
Hot tack is the ability of an adhesive or sealant layer to resist creep of the seal while it is still in a warm or molten state. Hot tack comprises two components: the melt strength of the seal layer at the temperature of the seal and the interfacial adhesion of the sealant layer to the opposite web. HSC materials provide greater hot tack at lower temperatures than do peelable film systems. HSC materials provide greater hot tack at lower temperatures than do peelable film systems. This is due in part to the dual role of a peelable film: polymers used in peelable resin systems must seal and peel, yet still provide structural support in order to form the film. Therefore, compatibility of the polymers is tightly controlled so that peel strength and film strength will be in balance. The incompatibility between the polymers in a blended system reduces the melt strength of the film to ensure peelability, therefore reducing the hot tack when compared with a single-polymer sealant layer. A necessary consequence of this is that, in the molten state, peelable resin systems are more viscous than HSC materials.
The primary functions of heat seal coating are to seal and peel. Typically, heat seal coatings do not provide the kind of structural support required of peelable resin systems. The lower the viscosity, the easier it is for the resin to flow and produce intimate bonds between the sealant layer and the other web, providing greater hot tack at lower temperatures. Another advantage is that HSC materials are widely available that feature lower activation temperatures than peelable film systems. Recent advances in peelable film technology, however, are closing the gap between conventional peelable resin technology and HSC materials. Figure 2 compares the hot-tack properties of HSC materials, conventional resin-based film systems, and an emerging peelable film technology for medical packaging.
SEAL TRANSFER FOR MEDICAL PACKAGING
Seal transfer provides a visual method of evaluating seal integrity, and hence offers an indication that a medical device contained in a package has remained sterile prior to use. Both peelable resins and HSC technologies provide some means of seal transfer, owing to the fact that both systems are typically cohesive in nature. Generally, however, seal evidence is more pronounced in coated systems.
Seal transfer makes it possible to detect differences between sealed and unsealed areas of the medical package. This allows for inspection of seal disruptions that could have resulted from sealing or sterilization, and thus could represent a compromise of the microbial protection afforded by the package. Advances in peelable resin technology have created seal transfer results comparable or superior to those of HSC systems. Figure 3 illustrates seal transfer of a blue-tinted peelable resin system and an HSC material.
COST-EFFECTIVENESS IN MEDICAL PACKAGING
When determining total medical packaging cost, one must consider both the hard (unit price, sterilization cost, packaging/shipping) and soft (machine efficiencies, sterilization time, waste) constituents of a package. Medical packaging components, line speed, and method of sterilization all affect the total product cost. The chart shown in Figure 4 indicates that peelable films offer a good balance between price and performance; HSC materials can be tailored to "fit" almost any point of the curve. Although using a coated material may have a higher material cost than conventional peelable film systems, the total cost can be equivalent once machine and sterilization efficiencies are taken into account.
FUTURE TRENDS IN MEDICAL PACKAGING
Recently developed peelable resin technologies are demonstrating significant improvements in performance. Peelable systems now entering the marketplace have been designed to peel through a cohesive failure mode, reducing the influence of seal-formation conditions on peel performance and providing seal transfer that is comparable to that of HSC materials. Seal ranges and hot-tack properties are also improving. These advances will enable peelable systems to run on the kind of rotary or continuous equipment that was previously the exclusive domain of HSC materials. In addition, the near future should see peelable resin systems capable of sealing to a variety of rigid tray materials.
HSC materials are also evolving to meet the changing marketplace, with products being developed that are even more robust than current formulations. In addition, HSC materials can be designed for relatively small-volume applications, which is beneficial to new and evolving markets. Coatings with lower activation temperatures for use with high-speed equipment are also under development. Another area of ongoing research involves water-based coatings with improved resistance to liquids that can rewet the adhesive.
CONCLUSION
The purpose of medical packaging is to protect and preserve the sterility of a device throughout normal handling and storage. Therefore, developing effective medical packaging requires an understanding of all the elements involved.
Both peelable films and HSC materials used in medical applications provide for a microbial barrier—an essential attribute of medical device packaging. Additionally, with either type of packaging, gas and moisture barriers can be achieved by tailoring the package construction to meet the device requirements. Films and laminations employing resin-based peelable sealant layers can provide excellent resistance to liquids and greasy materials. By contrast, water-based HSC materials, which can rewet, are less than desirable for such applications.
Peelable Films |
When to Use
|
Table III. Applications guidelines for peelable film packaging materials. |
Heat-Seal-Coated Materials |
When to Use
|
Table IV. Applications guidelines for heat-seal-coated packaging materials. |
Tables III and IV provide an overview of areas in which each technology is best suited. Peelable film systems tend to be price competitive and well suited for high-volume applications. At present, the selection of mating webs is limited because the current technology is sealable only to similar, polyethylene-based materials.
Autoclave sterilization is not recommended for peelable resin systems since most are based on polyethylene. However, radiation and EtO are effective means of sterilizing peelable film packages. All major methods of sterilization can be used with HSC substrates. Applications requiring short dwell times are best suited for HSC materials, which generally have better hot-tack characteristics than peelable films, although recent advances in peelable technology are narrowing this gap. HSC materials offer greater variety and can generally be tailored more easily to specific applications. The seal-transfer characteristics of HSC systems are also typically superior to those of current peelable resin systems.
There are many factors to be considered when specifying materials for a medical package. One needs to evaluate the whole process, from the product and its barrier requirements to packaging equipment and processing speed to method of sterilization. The type and speed of equipment to be utilized will be a determining factor when selecting materials. Package characteristics such as seal transfer and hot tack must also be evaluated. Because both HSC materials and peelable resin systems have broad applicability to medical packaging, it is critical to examine the total requirements of a device before selecting package materials.
BIBLIOGRAPHY
Barcan, D. "Using a Seal Matrix to Optimize Package Seal Variables." Medical Device & Diagnostic Industry 17, no. 9 (1995): 112–122.
Hall, R. "Developing a Medical Package." Medical Device Technology (January/February 1993): 23–27.
Komerska, J. "Peelable Coatings for Device Packaging: Today's Requirements and Future Trends." In Proceedings of the TAPPI 1979 Disposable Medical Packaging Seminar (Atlanta: Technical Association of the Pulp and Paper Industry, 1979), 12–15.
Merritt, J. "Comparative Dynamics of Rotary vs. Platen Heat Seal Systems." Packaging Technology & Engineering (July 1999): 28–30.
Spitz, J. "Peelable Seal Coating Systems—Considerations for Seal Integrity Validation." In Medical Design & Manufacturing West 1997 Proceedings (Santa Monica, CA: Canon Communications, 1997), 103-1–103-9.
Glenn Petrie is senior product specialist at Rexam Medical Packaging (Madison, WI), where he is a member of the product and process development team charged with developing coated products for Tyvek, papers, and film laminations. Rexam is a manufacturer and converter of packaging materials for the medical device and pharmaceutical markets.
Return to the MDDI January table of contents | Return to the MDDI home page
Copyright ©2000 Medical Device & Diagnostic Industry
About the Author(s)
You May Also Like


