Boston Scientific, Johnson & Johnson, Cook Group, Covidien, and Abbott have submitted requests for changes or clarifications to FDA's draft guidance on the global unique device identification database.
January 16, 2014
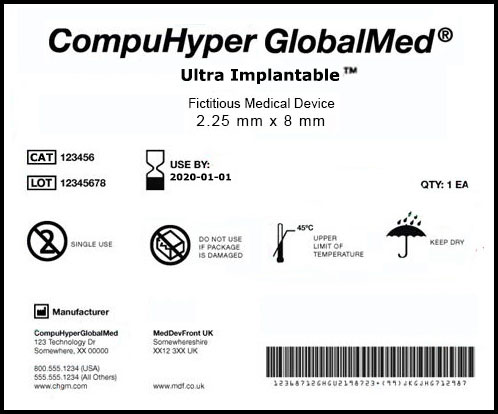
By Jim Dickinson
Five medical device companies and a trade association asked FDA in late November for significant clarifications and improvements to an agency draft guidance on the global unique device identification (UDI) database. All of the comment letters voice support for the agency’s efforts while asking for many clarifications and changes.
Dawn Fowler of Endologix will provide expert insight on UDI implementation at MD&M West in Anaheim, CA, February 10–13, 2014. |
In its comment, Boston Scientific said it supports FDA efforts to develop UDI systems for medical devices and associated tools.
“A clear and accurate guidance for industry regarding the Global Unique Device Identification Database will assist device labelers and FDA in obtaining consistent and accurate data,” the company said. “However, in reviewing the detail of the guidance, further clarity is necessary to enable device labelers to adequately prepare submissions in a timely and least burdensome manner.
Moreover, there are inconsistencies between the Unique Device Identification final rule and the draft guidance creating further confusion and necessitating changes to this document and perhaps to the database.”
Other specific comments with detailed requests for clarifications or changes were submitted by Johnson & Johnson, Cook Group, AdvaMed, Covidien, and Abbott.
Dawn Fowler of Endologix will provide expert insight on UDI implementation at MD&M West in Anaheim, CA, February 10–13, 2014. |
—Jim Dickinson is MD+DI's contributing editor.
[image courtesy of FDA]
You May Also Like


