How to Make the Most of Packaging Trends
PACKAGING
October 1, 2008
|
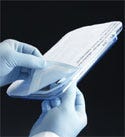
Clinicians and other surgical staff may prefer rigid trays with lids, such as this PETG tray with Tyvek. |
Medical device manufacturers have a lot to think about when it comes to medical packaging. OEMs must focus on satisfying the needs of healthcare providers, consider how to reduce packaging to cut costs and waste, ensure standardization, and accommodate drug-device combination products that require unique technology. Overcoming such obstacles may be daunting, but doing so is necessary. Addressing these challenges successfully increases a product's chance of adoption in the marketplace. In today's competitive business climate every edge gained is a positive. FDA requires that every product be packaged, so why not make the package a competitive advantage? This article explores the trends that medical device manufacturers face when they look for packaging and discusses how to approach those challenges.
Answering to Healthcare Providers
Success as a medical device manufacturer is defined as developing and marketing medical devices that fill a unique medical need or improve greatly upon existing technology. Such product development focus is targeted directly at patients and their specific needs. Additionally, OEMs must focus on improving the efficiency of the procedure or recovery time. It is important to recognize that patients are truly the focus of medical improvements and every upgrade should lead to better patient outcomes. However, in many situations the differences between medical technologies can be very slight, and the goal of the manufacturer is to sell more medical devices than its competitor.
One key driver is having your device selected by the healthcare provider more frequently than the competitor's device. Healthcare providers include the physician and nurse within a surgical suite, as well as other hospital staff. In many cases, they select the superior medical technology. But what happens when they have three similar devices to choose from? They have to make decisions based on other factors. One of those factors is the packaging that protects the medical devices.
|
In a survey sponsored by the Institute of Packaging Professionals (IoPP) and completed by members of the Association of Perioperative Registered Nurses (AORN), critical insights can be drawn to better understand the needs and preferences of healthcare providers. One of those preferences is the type of packaging. Of those surveyed, 60.4% preferred a formed rigid tray with lid, while 19.3% preferred a flexible pouch. In addition, 13.9% preferred a header bag, and 6.4% chose a formed flexible package with lid. Additionally, 84.4% of nurses said they wanted a double-barrier sterile system for packaging of medical devices.
Some of the most important factors with respect to packaging in this survey were the ability to read the label and the ability to quickly open the package during a surgical procedure. When identifying the key drivers to product selection and use when evaluating the label, it was noted that the sterility indicator and expiration date were considered the most important factors in product selection. Obviously, there are general labeling requirements that need to be addressed, and OEMs must put an emphasis on label designs that enhance such preferences.
Additionally, a tray that has product snapped into a cavity causes the fewest reoccurring problems. The highest reoccurring problems for standard medical packaging reported are package material tears and peeling the corner of a pouch. Additionally, 98.7% of those surveyed indicated that they inspect the package prior to opening, while 60% inspect it after they have opened the package. Flipping or dumping product into the sterile field is a common practice and is considered acceptable by 57.5% of those surveyed.
The takeaway message from this survey is that not all packaging is created equal. Success depends greatly on user preferences. A package that is designed with the specific healthcare user in mind will help drive market share, especially if competitors are not taking packaging seriously.
Pressures to Turn Green
|
Sterilization concerns must still be addressed for bioplastics to be used in medical devices. |
Sustainable. Green. Eco-friendly. These are terms that we hear on a daily basis. The pressure to reexamine practices with an eye for efficiency continues to gain momentum. Companies are concerned about becoming better citizens of the natural environment and are focusing on the carbon footprint they leave.
As medical and pharmaceutical companies begin to evaluate their own green initiatives, one item being examined closely is packaging. Such discussions address multiple facets, either specific packaging materials or the practices of the supplier producing the packaging. Currently, there is a lot of R&D being conducted by the packaging sector to develop and launch greener products.
Many market spaces within the packaging sector have already started to push toward green materials. For example, the food packaging industry has been using NatureWorks polylactic acid (PLA) for a number of years. It is a popular material derived from corn. The material's allure is its quick biodegradable profile and compostable properties.
Many such materials are being used in the cold food packaging market. Bioplastic materials, however, in their current form, have some limitations with respect to impact strength and heat deflection properties versus traditional petroleum-based plastics. At this point many of the bioplastics haven't come far enough to evaluate for sterile barrier systems. Device OEMs, however, should be aware that the technology is moving forward quite rapidly and might soon fit the needs of noncritical packaging.
As technology continues to improve, some of these green materials will find their way into medical packaging. In the meantime, several large medical device manufacturers are starting to evaluate other reduction mechanisms. For example, firms are trying one very simple approach, which is source reduction. A company must evaluate current packaging configuration and determine whether it can reach the same product protection outcome if the gauge or some of the component packaging is reduced slightly. Such small changes can result in eliminating a significant amount of raw materials.
Many packaging systems are overengineered. Reducing the amount of packaging can also aid in controlling costs. One specific example is the common use of double-sterile packaging systems. Often, such a system can be reduced to a single sterile system if the correct design is employed.
Another approach to sustainability is to use recycled plastic, in most cases postindustrial recycled material. The practice has seen success in food and consumer products packaging for several years. The best applications for recycled material are in noncritical medical packaging, such as work-in-process trays, consumer medical packaging, and nonsterile medical packaging. It is most common in rigid packaging, specifically with materials such as HIPS and PET.
|
PETG pellets are often recycled into sheets that can be used for packaging nonsterile Class I or over-the-counter devices. |
There are basically two methods for obtaining recycled or reprocessed material. One is postconsumer recycled material (e.g., PET soda bottles). This material is difficult to obtain because of the high demand. The other source is postindustrial recycled material (the material scrap or process waste that comes off of packaging lines and is recycled back into the material stream). That material is much more readily available and presents an interesting opportunity for packaging.
One of the organizations pushing for adoption of recycled and reduced packaging is the Sustainable Packaging Coalition (SPC). SPC is an industry work group championing cradle-to-cradle principles. It is working toward transforming packaging into a system that encourages economic prosperity and a sustainable flow of materials. A few medical companies have become members of SPC, including Abbott Laboratories and Johnson & Johnson.
Meeting Validation with ISO 11607
One of the major challenges for medical device packaging engineers is getting a package system to market quickly. In many situations, packaging is given a very short time to get the package system aligned before submission to FDA. As a result, it is critical that engineers have a clear and solid process for validating packaging. Part of the 510(k) or premarket approval is validation of the medical device packaging system, to ensure that the safety and efficacy of the device are not compromised. FDA views package validation as a documented process that provides evidence of meeting predetermined requirements on a continual basis.
Over the past two decades, medical device packaging professionals have been developing ISO 11607:2003 “Packaging for Terminally Sterilized Medical Devices,” which was first launched in 1997 and then revised in 2006. The goal was to produce a globally harmonizing standard that connected ISO 11607 to EN 868-1 and created a common platform and language. The outcome was a useful standard for the medical device industry.
|
Prior to ISO 11607:2006, every manufacturer had to provide FDA with a 510(k); however, submission methods varied from one manufacturer to the next. ISO 11607:2006 bridged that gap. As a result, it created a road map to assist device makers with compliance.
To complement the release of the standard, AAMI updated and released guidance document Technical Information Report (TIR) 22:2007, “Guidance for ANSI/ AAMI/ISO 11607, Packaging for Terminally Sterilized Medical Devices, Part 1 and Part 2.” TIR 22:2007 is a real-world review of ISO 11607:2006, providing practical guidance for packaging engineers. TIR 22:2007 gets into the specific application details a package engineer faces. It provides insightful details to aid engineers who aren't trained in package engineering but have the responsibility of package system design and development. At many small-to-medium device manufacturing companies, product development engineers also wear the packaging hat.
One of the main hurdles for the committee that worked on harmonizing ISO 11607:2006 was the terminology involved in describing medical packaging. Doing so was critical to creating a platform of international acceptance. As a result, the following four key definitions were developed. The definitions can greatly assist in the correct use of ISO 11607:2006 and TIR 22:2007, as follows:
Packaging system. Combination of the sterile barrier system and protective packaging.
Protective packaging. Configuration of materials designed to prevent damage to the sterile barrier system and its contents from the time of their assembly until the point of use.
Sterile barrier system. Minimum package that prevents ingress of microorganisms and allows aseptic presentation of the product at the point of use.
Preformed sterile barrier system. Sterile barrier system that is supplied partially assembled for filling and final closure or sealing.
|
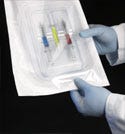
A tray inside a Tyvek pouch may be a user's packaging preference for particular surgical devices. |
Most medical device package engineers view ISO 11607:2006 and TIR 22:2007 as their bible for package development. Every decision a medical device package engineer makes with respect to design, material selection, sterilization, manufacturing, package testing, documentation, auditing, and supplier qualifications is made using the backdrop of ISO 11607.
Combination Products—A New Challenge
As medical technology continues to advance, packaging technology must also evolve to protect medical innovations and meet the new challenges presented by those advances. With the advances in biotechnology, pharmaceuticals, biologics, and nanotechnology, these technologies are now converging. One of the fastest-growing areas is the drug-device combination product arena. According to BCC Research, the total market for drug-device combinations worldwide was valued at $5.4 billion in 2004 and is expected to rise at an average annual growth rate of 13.3% to $11.5 billion in 2010. One of the most dominating combination products is the drug-eluting stent, which is predicted to grow annually at 11.5% through 2010.
Such growth has expanded the technical and regulatory requirements of packaging systems. To address the issues presented by regulating combination products, FDA established the Office of Combination Products (OCP) in December 2002. OCP has the broad responsibility of regulatory life cycle for combination products, but assigns primary regulatory responsibility and oversight of specific combination products to one of three product centers of FDA that most specifically apply. These three product centers are the Center of Drug Evaluation and Research, the Center of Biologics Evaluation and Research, and the Center for Devices and Radiological Health.
Combination products are typically a drug-device, biologic-device, or drug-biologic, as defined in 21 CFR 3.2(e). In general, they fall into the following categories:
Physically or chemically combined into one entity.
Copackaged.
Separately provided cross-labeled products.
Separate investigational products with proposed cross-labeling.
|
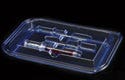
Drug-delivery devices such as syringes may have temperature and sterilization concerns that packaging should address. |
Traditionally, medical devices have fit into a pretty clear-cut category with respect to packaging and sterilization requirements. The key role for medical device packaging has been to provide product protection through the supply chain and sterilization, ultimately preserving sterility to the point of use. Combination products add complexity to the packaging system, creating new challenges such as shelf-life stability, solvent loss, package testing, and moisture and oxygen protection. They may also require additional package qualification found with pharmaceutical products.
With respect to combination products, barrier protection is a key component of successful packaging applications. When evaluating packaging options for rigid plastic tray applications, moisture barrier protection is found in a few different technologies. The most well known technology is an Aclar laminated to PVC or PETG. Typically, the Aclar structure is between 0.0006 and 0.003 in. depending on the targeted moisture vapor transmission rate (MVTR). The PVC/Aclar structures are popular in the pharmaceutical industry. PETG/Aclar is a growing area in combination products and biologics. One of the challenges, however, is the limited draw depth of the Aclar laminate. A thermoformed tray with an Aclar laminate can typically be no deeper than one inch.
|
A technician performs a quality check to evaluate packaging for particulate matter. |
Another moisture-barrier option on the market is a coextrusion that uses cyclic olefin copolymers (COCs). This technology has been developed and targeted toward the combination market. The coex is a core of COC, with skins of either PETG or polypropylene. One of the advantages of COC, because it is a coex and not a laminate, is that the depth of draw on a part can be up to five inches. In many rigid tray applications, flexibility in depth is important. The COC core typically starts at 0.005 in. and can range up to 0.040 in. depending on the targeted MVTR. COC is an amorphous material, with excellent clarity properties, and it can be sterilized by EtO and radiation.
Conclusion
As the competitive landscape evolves, medical device manufacturers need to consider any and all opportunities to gain a competitive advantage. Packaging is one of those avenues that is often overlooked. It is critical to listen to the needs of customers and find alternatives to meet those needs. Understanding packaging technology, international packaging standards, green initiatives, and design for the user all help device makers compete for market share.
Jason Crosby is the medical business manager for Plastic Ingenuity Inc. (Cross Plains, WI). Contact him at [email protected].
Copyright ©2008 Medical Device & Diagnostic Industry
About the Author(s)
You May Also Like




.png?width=300&auto=webp&quality=80&disable=upscale)
