Consider the Basics when Developing Packaging
PACKAGING
January 1, 2007
|
Rollstock, lids, and pouches are often used in sterile barrier systems to maintain sterility throughout a product's distribution cycle. Photos courtesy of OLIVER MEDICAL (Grand Rapids, MI) |
Behind every life-enhancing, state-of-the-art medical device is a packaging system that must perform flawlessly to maintain sterility throughout the distribution life cycle. The validation process for a medical device package should not be considered a single event, but a process of complex tasks that will adequately challenge and prove the efficacy of a sterile barrier system (SBS). Completion of these tasks ultimately demonstrates the packaging system's ability to maintain a sterile barrier until the point of use.
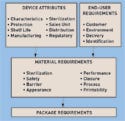
Innovation is hard to characterize and even harder to define. As device OEMs jockey for position on the leading edge of technology, engineers responsible for the packaging of medical products are challenged to implement innovative packages. Packaging systems changes are often based on enhancing device usability, product protection and delivery, and customer interaction. These initiatives can often fall short of expectations, however, because basic development steps are skipped in the quest for a hard-to-identify innovation target. To ensure packaging development success, OEMs should consider the importance of user requirements and thoroughly investigate the device characteristics. Ultimately, this research will result in the adequate specification of device packaging materials (see Figure 1).
Engineers responsible for packaging development are often asked to design and implement packaging systems under stringent cost considerations and product launch timelines. The internal pressures of cost, time, and resource limitations, coupled with the tempting familiarity of existing material knowledge and established specifications, often lead to insufficient material evaluation. The device attributes and packaging system requirements are overlooked, resulting in inadequately packaged devices. Continued use of this practice can produce potentially undesirable results, which over time can prove to be costly for end-users and device OEMs.
Underengineered packaging systems can result in package validation test failures. In addition, they can result in field issues warranting product withdrawals and customer perception concerns in terms of the quality, safety, and efficacy of a product. The most common issue surfaces when improper packaging materials or configurations fail during package validation. The short-term and most commonly used approaches to rectify this situation, such as overwrapping, bags, foam, and additional cartoning, lead to overpackaging of the device to accommodate the speed-to-market demands of a product launch.
Most device OEMs would admit that they have a packaging assembly process that closely reflects the one described above. Although the fix was implemented as a temporary one, it does not take long before line extensions and other product families are packaged in the same costly configuration.
|
Packages must be designed to meet material and packaging configuration requirements. |
Conversely, overengineered packaging systems are harder to identify, because it is commonly acceptable to rely on excess packaging to ensure device safety. Overengineered packaging tends to remain hidden or is overlooked. However, it affects the end-user in terms of aseptic presentation of the device, storage concerns, and waste disposal. Overengineered packaging affects device OEMs as well, driving up costs of materials, processing, and shipment of products. These hidden costs can add up over the life of a device. Ultimately, overpackaging affects the profitability of both device OEMs and medical care providers.
The ability to repeatedly hit the packaging system price-to-performance equilibrium target starts with an organized approach to gathering the design inputs that ultimately drive material requirements. Material and packaging configuration requirements should be set during the development phase of the project. To do so, engineers should consider the goal of a terminally sterilized medical device packaging system.
A packaging system should allow for sterilization, provide physical protection, maintain sterility up to the point of use, and allow aseptic presentation.1 Specific design attributes of a device also influence a packaging system's design and choice of materials. These include intended sterilization methods and the device's intended use, expiry date, transport, and storage.
Understanding User Requirements
Patients, physicians, and nurses are considered end-users of medical devices. Device performance is critical to the success of surgical procedures and other patient treatments. Problems arise when OEMs overlook the fact that a packaging system, which is part of device delivery, affects different individuals during the product delivery process. Most physicians do not acknowledge a package unless there is a problem that affects the patient's safety or the success of the surgical procedure. An OEM's field service and hospital staff, who read, inventory, organize, touch, and open devices, also should be considered end-users when developing a packaging system.
|
Figure 1. (click to enlarge) In a design requirements model, the device and end-user inputs influence the material requirements, which in turn affect the requirements of the final packaging system. |
Varying aspects of package interaction can be improved if user feedback and data are gathered from individuals that actually come in contact with the device package. Specific customer requirements created from these interviews can improve users' experience by reducing or eliminating the chance for mishandling and procedural errors. When evaluating customer needs, certain factors can be considered for field usage feedback.
Understanding the Customer. Vital to developing an innovative packaging concept is a firm grasp of who the customer is. Knowing how a product is used, the role it plays in a procedure, and the potential areas of concern if the package does not meet expectations can help to establish customer requirements. FDA's guidance on human factors, “Do It by Design,” is a good reference point.2
Understanding the Customer's Environment. The environment can affect how a packaged device is stored, transported through the healthcare facility, and used. Products used in a hospital emergency room will have vastly different user requirements than those used in a clinical facility.
Opening or Delivery of the Device. The device's delivery to the sterile field is one of the most important considerations when developing a packaging system. First, OEMs should determine the acceptable method in which a product should enter the sterile field. From there, it becomes clear whether a single- or double-barrier SBS should be used to facilitate the introduction of the product.
Device Identification. As it relates to labeling and the visibility of a medical device product, device identification is multifaceted. When considering packaging materials, determine whether there is a need for device visibility to allow for inspection and approval prior to opening of the SBS.
Determining Device Attributes and Requirements
The critical characteristics of a device may act as the packaging system design inputs to establish material requirements for that system. Device manufacturers should define what constitutes the successful performance of a device as well as consider the possible failure modes with regard to the packaging system performance requirements. The following considerations for product characteristics should be evaluated when developing the packaging material requirements. Although this list is not all-inclusive, these characteristics may be considered device-specific or may be used to justify the use of product family categories when developing a validation program.
Device Attributes. Characteristics related to size, shape, geometry, weight, intended shelf life, surface finishes, and protection requirements are examples of device attributes. Anything device related that may interact or be affected by the packaging materials in contact with the device should be considered.
Device Protection Requirements. Such requirements relate to specific environmental concerns and their effects, if any, on the performance of a device. It is important to consider specific requirements associated with exposure to oxygen, light, humidity, moisture, temperature, shock, compression, and vibration.3
Intended Shelf Life. A device's shelf life, if applicable, can affect material choice based on the ability of required performance attributes to withstand aging. If a device has a specific expiration date, the packaging materials must be able to perform sufficiently within the specified shelf life.
Manufacturing Requirements. These requirements should be determined by examining how the equipment and process of packaging a device affects the material's properties. Consider production volumes, product line expansion, manual versus automated loading, and available manufacturing technologies.
Sterilization. Sterilization processes affect packaging material requirements in two ways. First, specified materials must allow for sterilization (e.g., using a porous substrate to allow gas processing). Second, the packaging material must be able to withstand the stresses of sterilization, including multiple cycles at the process limits.
Sales Unit Configuration. The sales unit configuration determines whether a device should be single packed, multipacked, bulk packed, or have a combination of packages. A thorough evaluation and understanding of the dynamic environment is crucial to successfully determining material performance requirements.
Mode of Distribution. The environment in which a final device will be distributed also directly affects certain material performance requirements. The mode of distribution is as important as the sales unit configuration. Understanding whether a device will be palletized and shipped directly to an end-user, rather than sending a single device overnight, can affect a packaging system's suitability.
Regulatory Requirements. Packaging materials used can vary based on different global regulatory agency requirements. Device OEMs should consider specific market requirements when developing global packaging applications. For example, some regions do not readily accept materials that are commonly used in other parts of the world.
Specifying Adequate Packaging Materials
Determining user requirements and device attributes provides a device OEM with the knowledge required to evaluate and specify the most suitable packaging materials for an application. In turn, selecting the most suitable materials will help ensure that the package protects the device and avoids undesirable issues with waste, shipping, and user interaction. In addition, depending on the device's classification, compliance with various package test data requirements will be essential. Consider the following material requirements when specifying a package.
Sterilization. Material requirements can be affected by sterilization in two ways, as mentioned previously in this article. The chosen material must be compatible with the sterilization process and withstand its effects. OEMs should consult their suppliers for guidance on material suitability with regard to various sterilization processes.
Safety. As it relates to the biocompatibility and nontoxic classification of packaging materials, safety data are readily available for most of the commonly used materials in industry. Again, a firm's packaging materials provider can furnish more information.
Barrier Properties. Materials' barrier properties must demonstrate microbial barrier effectiveness. A few other commonly specified barrier properties provide increased protection from the effects of gas, light, and moisture.
Visibility and Appearance. Often considered aesthetic issues, visibility and appearance can negatively affect an end-user's experience and perception of a device. Understanding the environment, including the application, situation, and lighting, can affect the acceptance level of haze, gloss, and opacity.
Performance Characteristics. Also known as durability requirements, performance characteristics include material attributes such as puncture resistance, abrasion resistance, tear resistance, and flexural durability. Thickness, thickness range, tensile strength, elongation, basis weight, and bond strength are also performance characteristics. It is recommended that materials and their performance attributes be compared via results produced by standardized ASTM test methods.
Closure Seals and Opening Features. Such features facilitate aseptic presentation, a critical function of a medical device package. When determining the closure mechanism for an SBS, consider whether peelable or destructive bond seals with an opening feature should be used. Based on end-user requirements and device attributes, determine a suitable seal strength to allow for sterility maintenance while providing an easy-to-open package.
Processing and Manufacturing. The manner in which an SBS will be filled and sealed is determined by both processing and manufacturing. Similar to the manufacturing process, the material requirements are based on volume and available technologies. Certain packaging materials and assemblies are better suited for commodity-type high-volume and low-cost products rather than custom high-cost and low-volume products. The dynamics of processing and sealing will differ by method. For example, sealing a coated die-cut lid to a thermoformed tray introduces different stresses on the packaging materials than a form-fill-seal operation would.
Labeling and Printability. When determining how product identification will be applied to a packaging system, labeling and printability become critical considerations. Different types of direct-printing processes can produce varying results based on material structures and surfaces. Labeling of packaging systems can also affect material performance in terms of porosity reduction and adhesion difficulties.
Availability. OEMs often overlook materials' availability. The manufacturability, combined with the long-term availability requirements, of a material option should be considered when developing packaging assemblies. The magnitude of material qualification and packaging system validation work that is required to bring a product to market further demonstrates the importance of this consideration.
Conclusion
Developing innovative medical device packaging starts with the consideration of user requirements, which is then coupled with a thorough investigation of device characteristics. The successful completion of these two initiatives ultimately results in the adequate specification and usage of packaging materials. The information needed to adequately specify a packaging assembly is readily available. Implementation starts with understanding the need for the information, how to acquire it, and how to use it once it's gathered.
The most important aspect to consider is gathering user feedback through focus groups, field visits, trade shows, etc. Interaction with customers must be an active initiative to uncover and acquire actual real-world issues and problems. In terms of device attributes, internal teams understand the device better than anyone. The attributes, characteristics, and specific information must come from internal resources to ensure a thorough investigation has been completed.
Finally, considering information gathered from customers as well as looking at device attributes will help an OEM specify adequate packaging materials that will suitably perform in the intended application. Working with material suppliers to identify the material requirements will be more effective if the specifics related to user needs and device attributes have been identified. Following a process like the one described in this article allows for a methodical review of packaging requirements. In turn, OEMs will have more time and attention to focus on innovative medical device packaging.
Randall Troutman, CPP, is a principal engineer for Oliver Medical (Grand Rapids, MI). He can be contacted at [email protected].
References
1. ANSI/AAMI/ISO 11607-1:2006, “Packaging for Terminally Sterilized Medical Devices—Part 1: Requirements for Materials, Sterile Barrier Systems, and Packaging Systems” (Arlington, VA: AAMI, 2006).
2. “Do It by Design” [online] (Rockville, MD: FDA, CDRH, 1996); available from Internet: www.fda.gov/cdrh/humfac/doit.html.
3. Erik Swain, “Building Better Barriers” Medical Device & Diagnostic Industry 27, no. 10 (2005): 90–94.
Copyright ©2007 Medical Device & Diagnostic Industry
About the Author(s)
You May Also Like



.png?width=300&auto=webp&quality=80&disable=upscale)
