Active Packaging: Improving Shelf Life and Stability
Active scavenging films are shown to be viable alternatives to traditional gas flushing and purging methods for removing oxygen and moisture.
PACKAGING
|
|
Medical devices can be compromised by the presence of oxygen (O2) or moisture during sterilization and during the shelf life of the product. Manufacturers of devices and pharmaceuticals have implemented a variety of in-line procedures to reduce O2 and moisture levels to improve product integrity.
To date, in-line options for removing O2 and moisture in combination with a desiccant within packages are limited to vacuum packaging and nitrogen or argon purging and flushing. These procedures can be costly and inefficient for the manufacturing cycle. They can also often be unreliable and difficult to monitor from a quality assurance perspective and may not facilitate the complete removal of O2.¹
Active packaging systems are increasingly employed in medical devices, pharmaceuticals, and foods.²,³ Such systems scavenge target gases within the package and improve product shelf life and safety.4–6 O2 absorbers that are part of active packaging systems provide an alternative to vacuuming and gas-flushing technologies.7
Johnson & Johnson (J&J) Sterile Process Technology (SPT) continuously investigates methods to improve the shelf life and stability of sterilized medical devices. SPT collaborated with CSP Technologies (Auburn, AL) to determine whether its active scavenging films could be a viable alternative to traditional gas flushing and purging methods and to confirm CSP product claims that the scavenging films consistently remove O2 within a confined space to levelsTwo scavenging films of particular interest are CSP-1940 and CSP-1941. CSP-1940 scavenging film removes O2 and moisture, whereas CSP-1941 absorbs only O2. The mode of activation for O2 scavenging for both CSP scavenging films is ultraviolet (UV) radiation, and the activation equipment and process are easy to implement and validate.8 These films have been incorporated into new drug applications in both the United States and the European Union. SPT evaluated whether using CSP-1940 and CSP-1941 scavenging films for terminally sterilized pouches could provide performance and operational benefits comparable to current gas-purging systems. This article presents the outcomes of three investigations that were conducted to evaluate the feasibility of the CSP scavenging films for use in sterile packaging applications, as outlined below: • Evaluate effectiveness of CSP-1940 and CSP-1941 scavenging films to reduce O2 levels• Evaluate feasibility of using a traditional terminal sterilization process such as gamma irradiation to activate the CSP-1940 and CSP-1941 scavenging films and compare the films' performance to those using UV-radiation activation.• Ensure the effectiveness of CSP-1941 scavenging film in terms of UV-radiation activation after the scavenging film is exposed to an ethylene oxide (EtO) sterilization cycle. Technology CSP's core technology focuses on a patented three-phase polymeric system that generates a microarchitecture of one polymer within another. By combining two immiscible polymers with a particulate, CSP creates a network of interconnected channels that facilitate the controlled transport of small molecules throughout the blend.2,8,9 Uniformly dispersed, the pathways allow the diffusion of volatile substances into, out of, or through the material, enabling it to be custom formulated to absorb moisture, gases, odors, and aldehydes; release nutrients, biocides, flavors, or fragrances; or modify the transport properties of particular plastics.1–3,6,8–10 Many commercially available products today use this three-phase polymeric system in protecting and enhancing the products' useful life.6
|
Figure 1. (click to enlarge) Inactivated scavenging film (top left) and activated scavenging film that has turned brown (bottom left) upon O2 absorption; EMCM employed in the CSP O2 scavenging films (blue). |
The CSP scavenging films' polymer system is a blend of components. The first is an oxidizable base polymer, poly(ethylene methylacrylate cyclohexenyl methylacrylate) (EMCM; see Figure 1).8 The second component is a masterbatch consisting of a transition metal catalyst and a nonmigratory photoinitiator, in addition to a channeling agent and a base polymer.8 The nonmigratory photoinitiator allows the packager to initiate the O2 scavenging mechanism just prior to filling, which maximizes the capacity of the active scavenging component.¹¹
Upon UV-radiation activation, the polymer system of the CSP scavenging films absorbs residual O2 (via irreversible bonding) in the package headspace and additional O2 that may permeate through the primary packaging barrier.¹¹ The mechanism in which the polymer system absorbs O2 is as follows: 1. Activation energy is required for EMCM to react with O2. 2. Energy for the reaction is provided by UV radiation, which initially excites the photoinitiator. 3. A transitional metal catalyst helps initiate a chain reaction with EMCM, in turn making it more reactive with O2. The CSP scavenging films transition to the color brown over time as EMCM reacts with O2 (see Figure 1). This change of color provides a visual cue that the CSP scavenging films are functioning properly. More importantly, EMCM is unique in that no degradation products are generated as a result of the O2-scavenging process.¹¹ Figure 1 also illustrates EMCM pendant groups in the polymer backbone network in CSP scavenging films. Methods and Materials CSP scavenging films' performances had not been characterized when employing gamma radiation activation or when employing UV-radiation activation post-EtO sterilization. Since the performances of the films were unknown using these specific activation methods, the length of the investigation was established to achieve and record very low O2 levels within the pouches. Below is a list of the materials and equipment used: • CSP-1940 O2/H2O scavenging films.• CSP-1941 O2 scavenging films.• Aluminum foil–lined pouches (7 × 7 in.).• DuPont Tyvek pouches (7 × 7 in.).• Tekni-Plex clear barrier films.• GE Silicone II household glue.• Rubber O-ring fixtures/spacer frames.• Heat sealer.• UV-radiation activation chamber (capable of activating CSP scavenging films up to 4000 mJ/cm2).• Hamilton 10-ml Gas-Tight syringe.• Mocon Pac Check 450 oxygen meter (and associated accessories).• MDS Nordion Gammacell 220 Research Irradiators (2).• SPT research EtO sterilization chamber.
|
Figure 2. CSP scavenging film and O-ring placed within pouch (top). Sealed pouch with applied silicone septum (bottom). |
Pouch Preparation and Procedure. Each of the three investigations used the same type of sampling pouch: a hermetically sealed aluminum foil–lined laminated polyblend film for reduced O2 permeation. An O-ring was placed inside each pouch to create adequate headspace for repeated sampling from the same pouch. Each pouch was affixed with a silicone septum on the exterior surface from which single or multiple headspace samples were taken. Figure 2 depicts the construction of the pouch with the silicone septum used for sampling. The following describes steps for pouch preparation and the procedures for the three investigations:
1. UV-radiation activation of CSP-1940 and CSP-1941 scavenging films:• Properly sized CSP scavenging films are activated using a calibrated UV activation chamber.• CSP scavenging film and an O-ring are inserted into the pouch.• Pouch is then heat-sealed.• Silicone septum is applied onto the pouch.• Replicate pouches (min. n = 2) for each sampling point are prepared.• Pouches are shipped to SPT for sampling O2 levels on a weekly basis using the oxygen meter.• Additional silicone is reapplied to the septum to ensure proper seal during sampling. 2. Gamma radiation activation of CSP-1940 and CSP-1941 scavenging films:• Properly sized CSP scavenging films and an O-ring are inserted into the pouch.• Pouch is then heat-sealed.• Silicone septum is applied onto the pouch.• Replicate pouches (min. n = 2) for each sampling point are prepared.• Established dosage for the investigation is 25, 35, and 45 kGy: Pouches are exposed to various doses of gamma radiation by timed placement inside gamma cells (decay factors and dose rates during time of exposure are 0.220/4.4 kGy/h and 0.516/10.2 kGy/h for the gammacells); controls (0 kGy exposure) for replicate pouches are also included.• Pouches are sampled for O2 levels triweekly using the oxygen meter.• Additional silicone is reapplied to the septum to ensure proper seal during sampling. Note that CSP instructs its customers to employ UV radiation to activate their scavenging films. SPT used a gamma radiation source to activate the CSP scavenging films during terminal sterilization.
|
Figure 3. Tyvek pouch with O-ring (top). CSP scavenging film and O-ring sealed within Tyvek pouch (bottom). |
3. Evaluate the effects that EtO sterilization has on the performance of the CSP-1941 scavenging film:
• Properly sized CSP scavenging film is inserted into a Tyvek pouch having a clear barrier film on one side (see Figure 3).
• Replicate pouches (min. n = 2) for each sampling point are prepared.
• Tyvek pouches are subjected to an EtO sterilization cycle: EtO sterilization cycle is a SPT nominal, vacuum-dry, production cycle in a research EtO sterilization chamber; controls (no UV-radiation activation) for the replicate pouches are also included; total EtO sterilization cycle exposure is ~25 hours.
• Tyvek pouches are subsequently express shipped to CSP.
• Tyvek pouches are opened to remove the CSP scavenging film.
• CSP scavenging film is immediately placed into the UV activation chamber.
• A piece of clear barrier film is placed over the CSP scavenging film to cover it during UV-radiation activation: This activity would simulate exposing the CSP scavenging film in a specially designed pouch used for EtO sterilization and simultaneously allows for an optimal transmission of UV radiation.
• CSP scavenging film and an O-ring are subsequently inserted into the pouch.
• Pouch is then heat-sealed.
• Silicone septum is applied onto the pouch.
• Pouches are express shipped back to SPT for sampling O2 levels on a biweekly basis using the oxygen meter.
• Additional silicone is reapplied to the septum to ensure proper seal during sampling.
Note that CSP-1940 scavenging film is not evaluated in this investigation owing to the molecular sieve adsorbing moisture during the EtO sterilization cycle. The EtO sterilization cycle's high humidity would diminish the capacity of the molecular sieve to adsorb moisture and in turn, reduce the ability of the CSP-1940 scavenging film to protect the package over time. Results and Discussion
|
Figure 4. (click to enlarge) UV-radiation activation results of CSP-1940 and CSP-1941 scavenging films. |
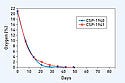
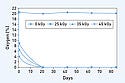
UV-Radiation Activation. The mean O2 levels are plotted versus the sampling day for the CSP-1940 and CSP-1941 scavenging films (see Figure 4). Both CSP scavenging films can decrease O2 levels to 2 scavenger. Overall, when either CSP scavenging film is properly sized to the container, O2 levels are consistently depleted toGamma Radiation Activation. The mean O2 levels are plotted versus the sampling day as a function of gamma radiation dose for the CSP-1940 and CSP-1941 scavenging films (see Figures 5 and 6, respectively). As early as Day 21, 0% O2 is attained and maintained throughout the investigation out to Day 84, regardless of the gamma radiation dose received. Moreover, for both CSP scavenging films, the results also indicate that the amount of initial remaining O2 post–gamma irradiation (Day 0.5) is directly related to the dose received at onset. Specifically, the data for both CSP scavenging films reveal a rate dependency; an approximate twofold decrease in initial O2 levels is attained from an approximate twofold increase in gamma radiation dose (25, 45 kGy; Day 0.5). This is eclipsed by an even more rapid depletion in O2 levels experienced between Days 0 and 0.5 for both CSP scavenging films.
|
Figure 5. (click to enlarge) Gamma radiation activation results of CSP-1940 scavenging film. |
However, additional O2 levels are not recorded intermittently (between Days 0.5 and 21) because the investigations are designed to evaluate the long-term behavior and performance of both CSP scavenging films as well as not to prematurely exhaust the pouch headspace for such purposes. Consequently, it is uncertain on which [earlier] day O2 levels actually reach 0% for both CSP scavenging films.
It can be projected, however, as evidenced by the rapid O2 depletion rate, that 0% O2 levels may be reached and maintained as early as one week. Future investigations using gamma radiation activation should confirm this. There is no evidence that the O2 scavenging is reversible. Improved results in repeatability are found within these investigations and are attributed to the resealing of the silicone septum immediately after sampling, as indicated by the low standard deviation values that are obtained (data not included for brevity). Finally, the results obtained from this investigation demonstrate that both CSP scavenging films can be effectively activated by gamma radiation and thus scavenge O2, maintaining low O2 levels over extended time periods in sealed pouches.
|
Figure 6. (click to enlarge) Gamma radiation activation results of CSP-1941 scavenging film. |
Significant performance advantages and unique sterilization options are created during a gamma irradiation sterilization process that activates the CSP scavenging films within a sealed medical device package. Two advantages in particular are an accelerated O2 depletion rate in comparison to the UV-radiation activation investigation (see Figures 4–6) and a dual-step approach that encompasses activating the CSP scavenging films at lower-than-set sterilization doses. In this dual-step approach, the first step would be to use a low dose of gamma radiation to activate the CSP scavenging films, thereby reducing bioburden levels. Once O2 is scavenged, a second sterilization dose can be applied to achieve sterility. This dual-step approach may enable a lower maximum dose of gamma radiation to be employed in achieving sterility, which could benefit products that may be compromised by high dose levels.
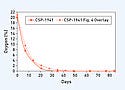
Use with EtO Sterilization Cycle. The mean O2 level is plotted versus the sampling day for the CSP-1941 scavenging film (see Figure 7). CSP-1941 scavenging film can decrease O2 levels to
|
Figure 7. (click to enlarge) UV-radiation activation results of CSP-1941 scavenging film post-EtO sterilization cycle. |
Using a packaging barrier material that is less UV-absorbing such as a nonconjugated, non-phenyl ring-containing polymer, may improve the UV-radiation transmittance through the barrier and increase the activation energy received by the CSP-1941 scavenging film. Nevertheless, the results clearly indicate the ability of CSP-1941 scavenging film to survive an EtO sterilization cycle. The data also suggest that the CSP-1941 scavenging film can be UV-radiation activated through a clear PET barrier of an EtO sterilization package such as a Tyvek pouch, depleting O2 levels to virtually 0%.
The O2-depleting capacity of the CSP-1941 scavenging film is not affected by the relatively higher humidity levels within the pouch resulting from the EtO sterilization cycle exposure. This is evidenced by comparing the results of the manufacturer's suggested UV-radiation activation method to the UV activation method post-EtO sterilization cycle (see the overlay in Figure 7). The rate of O2 depletion between these two investigations does not appear to be affected by the EtO sterilization cycle, which includes a high-humidity segment. This high humidity is often a critical segment of such EtO cycles. Applications The technology offered by the scavenging films may be used where O2 and or moisture is a problem in a closed environment. The removal of O2 and moisture can create an improved package environment during terminal sterilization or may simply extend shelf life of nonsterilized products. Although these investigations have focused on the efficiency of O2 depletion, the ability of the CSP scavenging films to deplete moisture simultaneously is also a very interesting aspect. The CSP scavenging films could be used where traditional desiccants are used or where purging is used to control oxidative effects of O2 or moisture. Possible uses of the CSP scavenging films include packaging for bioresorbable materials, biologics, and pharmaceuticals. Automation The versatility in commercial features for CSP scavenging films includes the following: • Sized to accommodate the application.• Provided in precut pieces to accommodate pick-and-place automation.• Provided in continuous reels.• Heat-staked (laminated) to foil-lined pouches.• Provided with an adhesive backing.• Kinetics and total absorption capacity can be customized for size and performance to the final package.• Automation assistance can be provided by PMI Cartoning (Arlington Heights, IL); Harro Hoefliger (Allmersbach, Germany); and Siebler Romaco (Karlsruhe, Germany). Figure 8 illustrates the various delivery platforms of the CSP scavenging films, whereas Figure 8c depicts a method of automated film or label placement.
|
Figure 8. Delivery platforms: CSP scavenging film on reel (a); scavenging films as die-cut/adhesive-backed labels (b); automation of scavenging film (c). |
Conclusions SPT successfully evaluated two CSP scavenging films and concluded that both films exhibited the ability to reduce O2 levels consistently toThe medical device and pharmaceutical development process presents a formidable challenge to successful product launches. In this light, SPT undertook this investigation to better understand and characterize the benefits that the two CSP scavenging films can offer. These benefits include the following. Providing a More-Effective Means to Remove O2. Traditional O2 purging or flushing systems yield a broad range of residual O2 levels. The variability in residual O2 levels is mainly because of equipment and processes. As a result, these processes can be difficult to validate and control over time. CSP scavenging films consistently reduce O2 levels toStreamlining Manufacturing. Purging a package with an inert gas takes time and can extend the EtO sterilization cycle time of manufacturing and packaging. The CSP scavenging films can be readily added into a package. They can also be heat-staked or adhered to the interior of foil pouches.4,8 Multiple automation vendors have provided CSP scavenging film application modules onto traditional packaging lines. In many instances, current production lines can be modified to accommodate the insertion of the CSP scavenging films. References
1. JP Kerry, MN O'Grady, SA Hogan, “Past, Current and Potential Utilisation of Active and Intelligent Packaging Systems for Meat and Muscle-Based Products: A Review,” Meat Science 74, no. 1 (2006): 113–130.
2. JB Polin, “New Markets, New Formats for Desiccants,” Pharmaceutical and Medical Packaging News 9, no. 7 (2001): 40–45.
3. S Strathmann, S Pastorelli, C Simoneau, “Investigation of the Interaction of Active Packaging Material with Food Aroma Compounds,” Sensors and Actuators B: Chemical, 106, no.1 (2005): 83–87.
4. J Kaufman et al., “An Overview of Oxygen Scavenging Packaging and Applications,” Packaging Network.com (October 2000); Available from Internet: www.packagingnetwork.com/article.mvc/An-overview-of-oxygen-scavenging-packaging-an-0003.
5. ML Rooney (ed), Active Food Packaging, (London, Blackie Academic and Professional, Chapman and Hall, 2005).
6. A La Coste et al., “Advancing Controlled Released Packaging Through Smart Blending,” Packaging Technology and Science 18, no. 2 (2005): 77–87.
7. M Ozdemir and JD Floros, “Active Food Packaging Technologies,” Critical Reviews in Food Science and Nutrition 44, no. 3 (2004): 185–193.
8. J Markarian, “Packaging Gets Active: Additives Lead the Way,” Plastics, Additives and Compounding 6, no. 2 (2004): 22–25.
9. E Mathiowitz et al., “Novel Desiccants Based on Designed Polymeric Blends,” Journal of Applied Polymer Science 80, no.3 (2001): 317–327.
10. V Coma, “Bioactive Packaging Technologies for Extended Shelf Life of Meat-Based Products,” Meat Science 78, no.1-2 (2008): 90–103.
11. BR Rodgers and JA Solis, “Factors Affecting the Performance of New Oxygen Scavenging Polymer for Packaging Applications,” Journal of Plastic Films and Sheeting 17, no. 4 (2001): 339–349.
Ike Harper is manager, R&D, at Johnson & Johnson SPT (Raritan, NJ). Frederick Halperin is a scientist at J&J SPT. Bridget Lang is a quality control chemist at Salvona Technologies (Dayton, NJ). Michael DiCicco is a senior scientist at J&J SPT. William Abrams is vice president, business development for CSP Technologies (Auburn, AL), and Brian Fitzpatrick is program manager for the company. Deepti Gupta is senior scientist, R&D, and Peter Sagona is vice president, applications development, for CSP. All correspondence for this article should be directed to Michael DiCicco. Copyright ©2009 Medical Device & Diagnostic Industry
About the Author(s)
You May Also Like