Manufacturers of Class III medical devices are now required to submit unique device identification data to FDA’s Global Unique Device Identification Database. Here’s how to ensure the process goes smoothly.
September 25, 2014

Manufacturers of Class III medical devices are now required to submit unique device identification data to FDA’s Global Unique Device Identification Database. Here’s how to ensure the process goes smoothly.
By Denise Odenkirk
Starting September 24, 2014, manufacturers of Class III medical devices have been required to submit unique device identification (UDI) data for their products to FDA’s Global Unique Device Identification Database (GUDID). In the coming years, this requirement will extend to manufacturers of Class II and Class I devices as well.
To comply, many manufacturers are choosing to leverage UDI to achieve a more consolidated view of product data within their organizations. Having a holistic technology solution that generates the required Health Level 7 (HL7) Structured Product Labeling (SPL)-formatted submission data, transmits the message to the GUDID, and tracks FDA responses back to each submission is key to accomplishing that goal.
Here are five best practices to ensure success with the complex GUDID publication process:
Be Prepared
This may seem obvious, but GUDID submissions need to be approached with the mindset that you will submit data only when you know it’s perfect. Don’t rush to submit before you’re ready.
Understand Your Data Attributes
A critical requirement for success is to verify the superset of data attributes required by FDA and your commercial community, as well as the workflow and process requirements that will be needed at your company to source, maintain, and publish product attribute data to your recipients. Manufacturers need to understand the specific data attributes FDA requires and the format in which the data needs to be stored for submission to the agency.
As an example, the UDI rule requires devices to be labeled as either “containing natural rubber latex or dry natural rubber (21 CFR 801.437)” or “not made with natural rubber latex”. Knowing that this is a requirement upfront will allow manufacturers to set up their data repositories appropriately and avoid missing data elements or incomplete submission records.
Verify Your Data
Once the data is ready, manufacturers should verify their data prior to submission to FDA. Submission simulators can be helpful in this regard. For example, FDA requires that the product attribute for sterilization method be included for all disposable Class III products. A submission simulator can validate that the appropriate data attributes are populated based on product classification type. Simulators allow users to see where FDA may have issues with their data before submitting to the test and production FDA environments. This saves time and effort because FDA may take several days to review and return failed submissions. A simulator allows users to immediately see which data attributes could fail, so they can be addressed ahead of time.
For example, all of the following have caused submissions to be rejected:
PMA number not 7 digits.
Labeler Dunn and Bradstreet Number (DUNS) not 9 digits.
“Kit Product” is a required field.
“Device Identifier Publish Date” is a required field.
Contact phone numbers must follow prescribed format.
Improper Global Medical Device Nomenclature (GMDN) term code.
Nonmatching DUNS numbers (DUNS number xxx is not a valid submitter for DUNS number yyy).
FDA listing and supplement numbers incorrect.
GMDN and FDA preferred term codes don’t align.
Understand the Submission Process
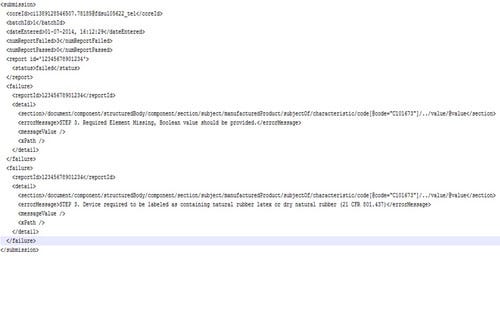
Once the data is prepared and ready, it’s time to submit to the FDA test environment. Once FDA approves the data, it can be submitted to the production GUDID. If the data is prepared properly, these last two steps should be simple. Manufacturers need to be prepared to receive and decipher the acknowledgement messages from FDA. The key to being properly prepared in this step is understanding what needs to be done to correct the errors as you receive the FDA acknowledgements. Figure 1 shows an example of an FDA failed acknowledgement with an embedded error message that shows the approved product labeling indicates the product contains rubber latex; however, the submission to FDA did not flag this appropriately on the submission.
|
Figure 1. Shown is a FDA failed acknowledgement with an embedded error message that shows the approved product labeling indicates the product contains rubber latex. |
Track Data Acknowledgements Closely
Acknowledgement receipt is the final critical component of any GUDID solution. Each submission transmitted to FDA is individually tracked, and all acknowledgements are captured and associated back with the submission data. The submission data, the human readable representation of the submission, and the acknowledgements become the revision-controlled UDI regulatory record for that product.
Denise Odenkirk is senior director of industry solutions for healthcare supply chain provider GHX.
[main image courtesy of STUART MILES/FREEDIGITALPHOTOS.NET]
You May Also Like