Smith+Nephew Nabs 510(k) Clearance for AETOS Shoulder System
The device is indicated for both anatomic and reverse total shoulder arthroplasty.
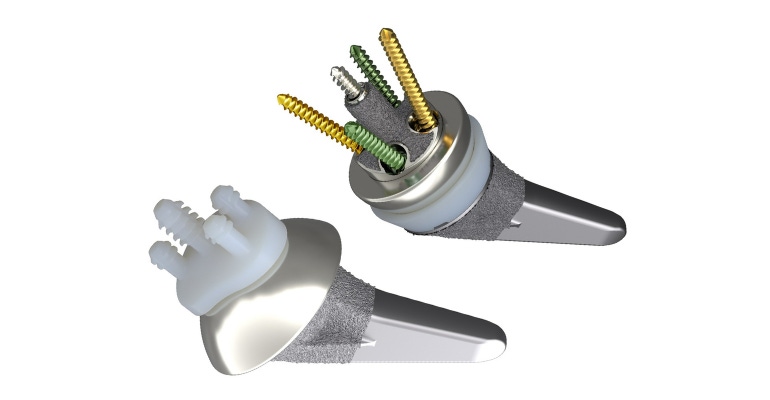
Smith+Nephew recently announced FDA 510(k) clearance for its AETOS Shoulder System, an anatomic and reverse shoulder replacement designed to restore range-of-motion and minimize arthritic shoulder pain for patients. The replacement features the AETOS Meta Stem, which is designed to maximize stability, preserve bone, and maintain patient anatomy, according to the company. The 510(k) clearance covers indications for both anatomic and reverse total shoulder arthroplasty.
Within the operating room, Smith+Nephew said that the system allows to intraoperative flexibility, including fewer steps for conversion and fewer instruments for primary anatomic and reverse implantation. Dr. James Kelly, a shoulder and elbow specialist at California Pacific Orthopaedics in San Francisco, and a AETOS system design surgeon, noted that the device is designed with a press fit, and is bone conserving. Additionally, he said the device offers maximum flexibility to reconstruct the humerus and glenoid.
"The AETOS Shoulder System was designed to be a cutting-edge press fit, bone conserving, convertible humeral stem,” Kelly said. “The system is designed for surgeons who desire maximum flexibility to reconstruct the humerus and glenoid, using an efficient and intuitive system that helps prioritize patient outcomes."
The shoulder system is the company’s latest device in its expanding upper extremity portfolio.
"Receiving FDA clearance for the AETOS Shoulder System is a major milestone for Smith+Nephew,” said Brad Cannon, president global orthopaedics for Smith+Nephew, in a press release announcing the clearance. “This platform is the culmination of years of research and development and represents our commitment to providing healthcare professionals with the best possible technology for their patients. We are confident that this platform will further evolve the standard of care for shoulder surgery and are excited to see the impact it has on patient outcomes."
About the Author(s)
You May Also Like



.png?width=300&auto=webp&quality=80&disable=upscale)
