iTotal CR Knee Replacement System from Conformis Earns Updated Rating from UK Panel
The jump from a 5A rating to a 7A rating is based on seven years of implant performance data.
March 2, 2022
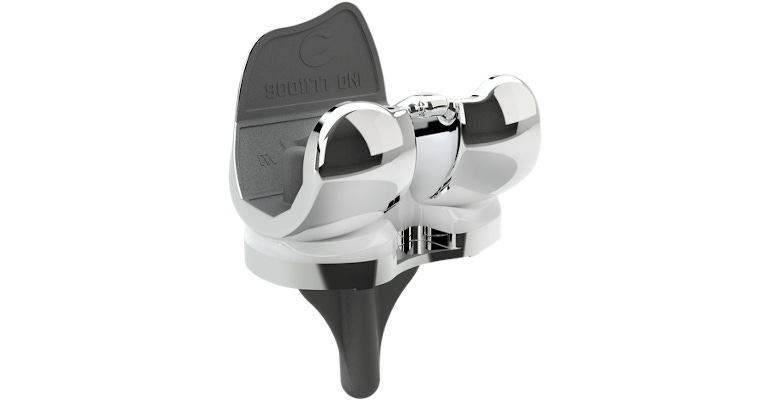
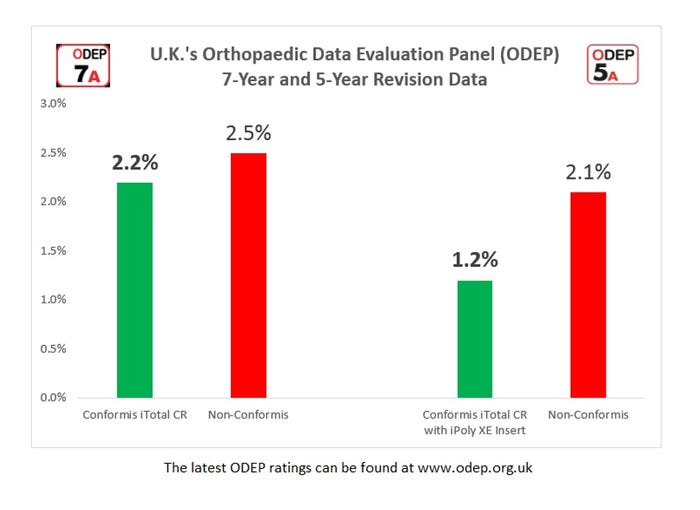
The United Kingdom's Orthopaedic Data Evaluation Panel (ODEP) has granted a 7A rating to Conformis Inc.'s iTotal CR (cruciate-retaining) knee replacement system. The rating is the result of an independently verified assessment of the system's performance over a seven-year period, superseding its previous 5A rating. According to Conformis, the iTotal CR knee has shown low rates of revision, which means removal and replacement of at least part of the original implant. Another of the company’s products, the iTotal CR knee with Poly XE insert, has received a 5A rating from the agency.
More than 70 surgeons in the UK to date have implanted more than 1,300 iTotal CR implants with seven-year cumulative revision rate of 2.2 percent versus 2.5 percent for all other knee replacements in the National Joint Registry (NJR) at the same seven-year postoperative time point, the company shared in a news release.
“The updated performance ratings complement what I have already learned from my years of using Conformis knee implants—namely, Conformis provides superior fit and extremely high-quality outcomes,” said Mr. Stuart Roy, Consultant Trauma and Orthopaedic surgeon at Spire Hospital Cardiff and Royal Glamorgan Hospital in Cardiff (South Wales), UK, in the release. “I continue to recommend the Conformis iTotal CR knee for many of my patients.”
ODEP ratings help clinicians identify implants that comply with national clinical best practice guidelines. ODEP consists of orthopedic surgeons and experts in the UK that evaluate data from hip, knee, and other joint implants. The agency generates a rating that measures performance in key areas, including survivorship, and it provides the UK’s National Health Service with an approved list of products that meet the revision rate standard set by the National Institute for Health and Care Excellence (NICE) in the UK.
“The latest ratings from ODEP provide continued independent validation of the Conformis design philosophy and our unique, customized manufacturing capabilities,” stated Mark Augusti, chief executive officer and president of Conformis, in the release. “Our goal is to provide a better experience where it matters most – in patients’ lives – and the ODEP rating reflects the high performance of our products at NHS facilities throughout the United Kingdom.”
Last September, Conformis shared results of a retrospective study of procedures using iTotal CR knee replacement implant considering implant survivorship, patient satisfaction, and functional outcomes. The study, “Patient Satisfaction, Functional Outcomes, and Implant Survivorship in Patients Undergoing Customized Cruciate-Retaining TKA”, was published online in the September 2021 edition of Journal of Bone and Joint Surgery. The primary results of the clinical study include:
Implant Survivorship – 98.5% - 8 revisions (representing 1.5% of the 540 implants reviewed) at a mean follow-up of 2.8 years (ranging from one month to seven years post surgery)
Patient Satisfaction – 89% reported being either satisfied or highly satisfied
Functional Outcomes – high mean score of 82 (range: 34 - 100) using the Knee injury and Osteoarthritis Outcome Score for Joint Replacement (KOOS-JR) questionnaire.
“This study was one of the largest retrospective studies completed in our history and is another example demonstrating that our technology drives high patient satisfaction, great functional outcomes, and high implant survivorship” said Augusti in a news release.
About the Author(s)
You May Also Like

.png?width=300&auto=webp&quality=80&disable=upscale)
