Workgroup Technology Corp.,
July 1, 1997
Profile
Design Control Regulations
Simplified with PDM Software
With FDA's new design control regulations into effect as of June 1, medical device manufacturers are quickly moving to adopt document and process management systems to control design documents. While instituted primarily for the purpose of meeting regulations, these systems are having additional benefits. Companies are finding that they are increasing their overall operating efficiencies as well as shortening their time-to-market cycles.
One company that has already begun meeting the new regulations is Medrad (Pittsburgh), a leading manufacturer of fluid-delivery and magnetic resonance imaging products. While the business has always been well run, over the years different departments within it struggled to manage the voluminous amount of data that were stored during the design and development processes.
For example, its document control group was using an aging Rbase database designed in the 1980s to store current files, while older versions were stored off-site at a cost of several thousand dollars each month. Rbase provided no control over historical files because it didn't contain the files themselves, only information about them. Further, it offered few data validations and provided no links to files.
At the same time, Medrad's engineering design and services group, which manages all of the company's electronic product documentation including Pro-Engineer CAD models and mechanical drawings, recognized an opportunity to improve the management of its current and historical files.
FDA, ISO Requirements
At a higher level, Medrad wanted to more efficiently meet the increasingly stringent regulations required by FDA. In addition, it sought to simplify the process for receiving ISO 9001 certification.
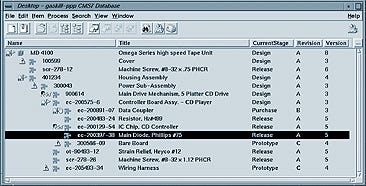
FDA requires that medical device firms maintain a device master record (DMR), which is the recipe for how the product is to be made. It contains all the product documents outlining the manufacturing processes and specifications. FDA also requires a device history record, which is a record of all the steps actually performed to produce the product.
All documents in the DMR must be under revision control. It is not unusual for FDA to check to ensure that the right configuration of documents was used on a particular date to build a specific device identified by its unique serial number or lot number. Maintaining all these documents and configurations is very difficult to do manually. For example, in order to prepare a DMR for even a small product line, Medrad might need to spend six to eight weeks manually going through documents and distinguishing which ones would be required to build a specific configuration. Then, within a day, the configuration could become outdated because of changed parts or procedures.
ISO 9001 requirements also pose a challenge to device manufacturers. ISO 9001 requires companies to document all their procedures and prove that they are followed. It includes the management of product documentation and the control of the design procedures.
Given these demands, Medrad determined that a product data management (PDM) system was the best way of addressing them and formed a five-person team that drew up a list of 30 potential suppliers. That list was quickly whittled down to 15 based on the need for a solution that could accommodate diverse hardware, operating system, and database requirements.
Then, after participating in demonstrations and more research, the group focused on three potential suppliers. Medrad brought each of the three vendors in for a week and had 16 key users gain hands-on experience with the products. Based on the results of a user survey that followed these sessions, the company selected the CMS PDM system from Workgroup Technology Corp. (Lexington, MA).
Key Selection Criteria
According to Paul Kwiecinski, Medrad's PDM project manager, there were several key reasons for the decision. "We really liked Workgroup Technology's focus on tool integration," he says. "For example CMS/Pro is a tight integration between CMS and Pro-Engineer, which enables our engineers to manage the complex configuration of files generated during product design."
Kwiecinski continues, "We also appreciated CMS's graphical file structure browser, which allows users to see how information is organized. In addition, CMS was easier to use than competitive products. With CMS, Medrad could tailor the custom attributes screen with its own data without any programming. Another PDM product required all documents to be stored in its own proprietary format, whereas CMS could retain information in its native format."
From an information systems standpoint, Medrad liked CMS because it allowed the company to define the applications that launch and work with CMS. "At the time, we did not have a corporate standard for word processing," Kwiecinski says. "CMS enabled us to select different viewers based on the individual PC desktop."
Time-to-Market Savings
Today, Medrad has control over a broad array of files, including WordPerfect, Word, Excel, Lotus Pro-Engineer, and others. "Now, our users can go through CMS, look up the document they want, and obtain it," explains Kwiecinski.
"We estimate that our R&D and mechanical engineers save up to two days a month just searching for information. That's significant to our organization. We can obtain information directly at the desktop without making special requests. CMS also gives us a lot better control over the different versions of each of the files. We no longer have new versions in-house and older versions sent off-site."
Moreover, Kwiecinski adds, "When a user puts in a change request today, the drawings and documents are directly affected by the change. With CMS, associations are made from the change request back to the original control documents. This means that when users look at change requests, they are assured of looking at the original document, and vice versa. Now people can see that association; we never had that with our old system."
In the past, Medrad could not easily view older revisions of documents. "Now," says Kwiecinski, "we have quick access to all revisions, no matter how many times a document has been changed. That has proved to be very beneficial. Today we have an automated audit trail to more quickly and easily meet FDA regulations and ISO 9001 standards."
Meeting Regulatory Requirements
From a regulatory perspective, CMS has performed well. "FDA was impressed with our demonstration of how CMS would enable us to meet its regulations," Kwiecinski says. "When it saw CMS, FDA said our method would exceed its requirements. They had suggested that we keep a separate device master record for each configuration of our injectors, and we have more than 650 different configurations. CMS will accomplish this so we will be fully FDA compliant." CMS has helped Medrad maintain better documentation in its device master records and device history records. The same process that could have taken six to eight weeks can now be handled on-line in minutes.
"Overall we believe CMS will play an important part in helping us get our products to market more quickly," Kwiecinski says. "This is extremely important in the medical device market. We estimate that CMS will provide our engineers with at least 10% more time to do their jobs. In conjunction with a number of other programs, this should have a significant impact on reducing our cycle time."
For more information on PDM software from Workgroup Technology Corp., call 617/674-7629.
MPMN is actively seeking success stories like this. If your company has one to tell, please contact Managing Editor Ursula Jones at 3340 Ocean Park Blvd., Ste. 1000, Santa Monica, CA 90405; 310/392-5509.
You May Also Like


