Originally Published MDDI February 2006
February 1, 2006
CDRH Takes Reins on Controversial Approvals
|
Although FDA was in the crosshairs of critics in 2005 (see “Criticism, Reform on FDA Agenda,” below), that did not mean CDRH shied away from controversial decisions. “On many tough issues, CDRH deserves a lot of credit,” says Jonathan Kahan, a partner at Hogan & Hartson LLP (Washington, DC). “Despite the invective in some cases, [the center was] able to evaluate the issues using a scientific and regulatory approach.”
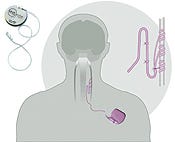
For example, CDRH approved Cyberonics Inc.'s (Houston) vagus nerve stimulator for treatment of depression, the first neurostimulation device allowed for that application. Despite the novelty of the treatment, the center was able to look past public criticism of the device and approve it based on the data. At the same time, CDRH took care to ensure it won't be used recklessly. It is limited to use in severely depressed patients who have not gotten “adequate response” from at least four other treatments.
The agency also allowed the return of silicone gel breast implants to the U.S. market, authorizing a product made by Inamed Corp. (Santa Barbara, CA). Silicone implants had been removed from the U.S. market in 1992, and a number of consumer groups lobbied hard for the exclusion to continue. But CDRH found scientific justification in allowing Inamed's product on the market, and the center issued the company an approvable letter in September 2005 with a list of conditions that must be met in order for silicone implants to return to the marketplace.
These decisions came despite enormous pressure to make decisions quickly, in order to meet the Medical Device User Fee and Modernization Act of 2002 (MDUFMA) performance goals.
MDUFMA goals have “taken a toll on reviewers,” Donna-Bea Tillman, director of the Office of Device Evaluation, told a crowd at the MassMEDIC FDA Update in December. We are overextended.” Focusing on the MDUFMA goals has prevented reviewers from participating in professional development, training, and guidance development, she said.
Although some in industry believe that the time pressure has caused CDRH to issue more not-approvable and not-substantially-equivalent letters rather than undertaking lengthy reviews, others give the center credit for tackling the toughest issues thoroughly.—ES
Copyright ©2006 Medical Device & Diagnostic Industry
You May Also Like