Using Acrylic to Lower Costs and Make Smaller Devices
As medical device prices increase and their sizes shrink, careful materials selection is becoming one way that OEMs can reduce costs.
COVER STORY: MATERIALS
|
Images courtesy of CYRO INDUSTRIES/ DeGUSSA (Parsippany, NJ) |
Mushrooming healthcare costs have been plaguing the United States for decades. The cost of standard medical care for the average family is estimated at $12,200 per year.1 As that price continues to move out of the financial reach of more and more Americans, the search for ways to curb costs is intensifying. To offset burgeoning costs, the healthcare industry is evolving. Two current trends will have a great effect on the medical device sector—the heightened focus on industrywide efficiency and the rapid explosion of home medical care. In response to these trends, medical device manufacturers must both streamline manufacturing strategies and adapt production processes to accommodate small devices used in home care. Critical to responding to both of these challenges is device material selection.
It has long been understood that materials directly affect product quality. But as belt-tightening and device shrinkage continue, device manufacturers are taking notice of the sometimes-subtle effect that materials can have on production efficiency and the bottom line. New requirements for medical device materials are driving the material selection decision in today's marketplace, and in some situations, acrylics can provide an answer. Factors to take into consideration include specialty material characteristics such as limited drug or fluid interactions in devices, high-flow capabilities, and ease of processing and chemical resistance. In addition, it is important to understand how material selection can serve as the foundation for breakthrough products with market dominance.
Heightened Focus on Process Efficiency
According to a Blue Cross Blue Shield report, about 20% of the rise in inpatient hospital costs can be attributed to medical technology, as can about 18% of the increase in outpatient hospital costs.2 As similar reports about the healthcare crisis appear, consumers, lobbyists, and legislators are demanding more cost-effective medical care and are pressuring manufacturers to produce quality devices more efficiently.
In addition, fierce competition, especially from global companies in low-wage regions, are forcing manufacturers to do more with less while material and fuel costs continue to rise. To survive, streamlined production processes are essential.
Unfortunately, in the face of this pressure, many manufacturers select materials based on price alone. Yet hidden costs downstream can create inefficiencies that result in higher total costs. For example, incompatible materials that are difficult to bond lead to high scrap rates that quickly drive up production costs. Similarly, materials that are not easily processed or that are not weldable with common techniques also increase costs.
There are several key points that manufacturers should discuss with their material suppliers to avoid being snared by hidden costs. These include material properties that enhance efficiency, other value-added properties, and partnership opportunities.
Efficiency-Enhancing Properties. Easy-to-process materials can streamline a production process by reducing cycle time and adding flexibility. Most plastic materials are easily injection molded and can be readily adapted to tooling designed for other plastics. For example, acrylic can be processed on equipment used for amorphous plastics such as styrene, acrylonitrile-butadiene-styrene (ABS), and polycarbonate. Flexibility, and thereby efficiency, can also be enhanced with materials that can be readily welded with a variety of techniques including radio-frequency, ultrasonic, vibration, spin, or hot-plate processes.
It is also beneficial to select materials that can withstand a variety of sterilization methods, including gamma, ethylene oxide (EtO), electron beam (E-beam), and STERRAD system techniques. Acrylic can withstand most sterilization methods. The exception is steam sterilization, which is used most often to sterilize reusable devices in hospital environments and exposes devices to very high temperatures. Because acrylic cannot generally tolerate temperatures above 180°F, it is better suited for disposable devices undergoing less-heat-intensive sterilization techniques.
Materials that are defect-free, consistent, and flow well (to properly fill out molds) can reduce waste. Another important consideration is the temperature at which materials are molded. Processes requiring higher temperatures require more energy, which can drive up production costs. Various plastics differ in this regard, with polycarbonates generally using more heat for processing than acrylic. Acrylic cools at mold temperatures of 120°–170°F, while polycarbonate cools at mold temperatures of about 180°F. In addition, acrylic requires melt temperatures between 400° and 480°F, versus a 572°F melt temperature for polycarbonate.
|
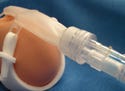
Figure 1. An acrylic-based multipolymer compound has high-flow properties and can be ultrasonically welded to make medical components such as fluid check valves. |
Such efficiency-enhancing characteristics often make acrylic a good choice for medical devices such as fluid check valves (see Figure 1). Materials with consistent melt flow can facilitate accurate molding because they eliminate short shots—devices that are not fully formed owing to improperly packed-out molds. They also reduce the risk of uneven molding, resin waste, and product rejects.
In addition, to create a leak-free seal in check valves, the housing and cover are often ultrasonically welded in an automatic assembly process. Because acrylic is rigid, it is well suited for this process. The same cannot be said for softer plastics, such as polyvinyl chloride (PVC) and most urethanes and olefins, because energy dissipates rapidly in these compounds.
Check-valve material resistance to gamma, E-beam, and EtO sterilization is also beneficial, as it enables them to be sterilized with an OEM's method of choice. Acrylic, as well as polycarbonate and copolyester, can prove useful. Of course, specific OEM circumstances determine which polymer is ideal. For example, OEMs using gamma irradiation may find acrylics preferable, because they offer strong color stability with minimal yellowing after sterilization.
Value-Added Properties. Materials with value-added properties can also reduce total manufacturing costs by eliminating the need for extra production steps or additives. For example, an acrylic-based polymer that is inherently static dissipative eliminates the need for antistatic coatings. Eliminating that step results in a shortened production process. Another example is polymer-liquid interactions with drugs, solutions, or body fluids that can enhance or impede the efficiency of a device. Materials that have a high attraction for blood or lipid solutions, for example, may cause inefficiencies in blood-handling devices if, in the application, blood components stick to the sidewalls of the device.
Opportunities for Partnership. As medical technologies and manufacturing operations advance, the number of factors to consider when selecting a material increases. This has led to a morphing of the traditional supplier-buyer contract into a true consultative partnership, in which OEMs seek guidance and expertise from materials providers. A partnership approach enables OEMs to leverage the experience of their vendors to identify possible future hidden costs, determine root causes of material production issues (e.g., whether the material or manufacturing process is to blame), and reduce scrap for leaner operations. Involving materials providers from the project onset can also help OEMs select the best material for a specific process, rather than trying to adapt the process to the material as issues arise, which can be both inefficient and costly.
Vendor expertise is valuable, for example, when evaluating material compatibility. Most plastic medical devices comprise several components that must be bonded or welded together, and so compatibility is critical. Consider the manufacturing of a filter device with a housing made of an amorphous material that is bonded to a membrane of a semicrystalline material. Without a strong seal, which ensures that liquids can pass through the device without leaking out, products are scrapped, and thus time, materials, and money are wasted. To avoid this inefficiency up front, several complex factors should be evaluated. Chief among these considerations are the materials' chemical compositions, melt-flow characteristics, and effective methods of bonding dissimilar materials.
In this case, difficulty could arise because amorphous materials, like acrylic, soften to a thick, viscous melt in high temperatures. However, semicrystalline materials, such as clarified polypropylene, melt like wax, changing from solid to liquid very quickly when heated. The two materials cannot simply be melted together, because amorphous materials do not truly melt. Moreover, bonding cannot rely on friction because liquefying semicrystalline material reduces friction. Therefore, an adhesive or alternative design would need to be employed for bonding. If such considerations are understood from the start of a project, hidden costs can be avoided later in the production process and greater efficiency will ensue.
Reducing Device Size
In addition to intensifying the need for process efficiency, the rising cost of healthcare has also led to an explosion in home care and, therefore, home-care devices. Shortened hospital stays are causing patients to take control of their healthcare. Accordingly, the market for home-use medical devices is expected to grow approximately 9% through 2008 and double in size by 2011.2 But self-administered medical treatments, at-home monitoring technologies, and ambulatory devices must be cost-effective, portable, more durable, and smaller than their counterparts housed in hospitals and doctors' offices.
To meet the demand for smaller and more-complex devices, OEMs can benefit by selecting materials with the following key characteristics:
Processability.
Appropriate weight and durability.
Chemical and lipid resistance.
Transparency and UV consistency.
Processability. As devices get smaller, the mold cavities used to make components decrease in size as well. Often, this results in a greater number of cavities in a mold, which complicates the molding process. To consistently fill these intricate molds and meet tight tolerances, manufacturers require high-flow materials with good processability. Better dimensional stability is also critical as device walls thin. And materials with high melt strength are desirable because they minimize stress concentration areas and provide good thickness distribution when thermoforming is involved. Although the ideal dimensional stability for an application depends on the level of rigidity required, most plastics used in the medical industry have good stability.
|
Figure 2. (click to enlarge) One study compared the spiral flows of various materials. Except where noted, the study used a melt temperature of 450°F and a mold temperature of 105°F. |
A common measurement of material processability is spiral flow, which measures the length traveled by polymer melt when injection molded into a cavity. In one study, shown in Figure 2, the spiral flow of an acrylic polymer compound was compared with those of a copolyester, a transparent ABS terpolymer, and two types of polycarbonate. In this case, the acrylic exhibited better flow and would be useful in devices requiring high-cavitation molding and thin walls. However, stiffer-flow plastics could be used to manufacture large devices with less-intricate molds, since they would not require high-flow materials.
Weight and Durability. Ambulatory devices and other technologies that are transported frequently require lightweight materials. Weight is a function of a material's specific gravity, which compares the material's density with the density of water. The lower the specific gravity, the more parts can be produced per pound of material, leading to a lighter finished device. But although weight is important, device OEMs must not sacrifice durability.
Devices used in the home are prone to unusual wear and tear and transportation damage. Damaged devices can be especially hazardous in home-care applications because they are not subject to regular inspection by clinicians. Therefore, home-care devices must be able to withstand abuse, which makes material durability critical.
The durability of plastics is measured using a variety of tests, one of which is the Izod impact strength test. Izod impact strength is defined as the energy required to break a material when a crack is formed. The results are measured in foot-pounds-per-inch of thickness. Certain types of plastics are more durable in a given situation than others. For example, while polycarbonates are generally highly impact resistant, they become brittle when exposed to lipids and chemicals. Acrylics are not as innately tough as polycarbonates, but they maintain their durability better after exposure to such substances.
Some applications, such as tubing, require flexible materials. In such applications, silicone, PVC, or polyurethane would be ideal, while acrylic, which is rigid, would not. In addition, each supplier may have several plastic grades with varying levels of impact strength. Manufacturers should evaluate specific project needs and different strength characteristics to determine the level of durability required.
Chemical and Lipid Resistance. At-home devices must also be especially resistant to pharmaceuticals, chemicals, and bodily fluids to avoid patient harm. This is especially true for respiratory-care products, a fast-growing segment of the home-care market. Respiratory-care devices include nebulizers and other inhalation therapy devices that often come in contact with harsh substances.
|
Table I. (click to enlarge) Chemical resistance of an acrylic compound and a lipid-resistant polycarbonate to lipids and isopropanol. Elongation retention after exposure to stress in the presence of a chemical measures chemical resistance. |
Table I and Figure 3 show data for two plastic materials—an acrylic-based multipolymer compound and a lipid-resistant polycarbonate. The materials were evaluated for chemical and lipid resistance using a commonly applied test. This test entailed placing each material under 2% strain and exposing them to isopropanol and lipids. Tensile elongation was measured before and after exposure to detect any changes in ductility, which would indicate the risk of breakage. Although the acrylic was less ductile than the polycarbonate prior to exposure, it maintained its ductility after contacting the substances. However, the polycarbonate became much more rigid and prone to breakage after exposure.
|
Figure 3. (click to enlarge) Lipid resistance (top) and isopropanol resistance (bottom) of an acrylic multipolymer compound and a lipid-resistant polycarbonate. |
Continuous positive airway pressure (CPAP) equipment is currently leading the respiratory-care market, with growth rates of 15–18%.3 Because of their frequent contact with harsh substances, these devices require materials that are tough, bondable, and easy to process. Most plastic compounds exhibit these qualities and are contact-compatible with a range of human fluids and pharmaceuticals, which facilitates device approval by FDA (see Figure 4). Chemical resistance is similarly required for point-of-care insulin treatment and home-care dialysis devices, which are in frequent contact with blood and pharmaceuticals. This may rule out materials that are not resistant to the specific chemicals used in an application.
Transparency and UV Consistency. For small diagnostic devices that use optical technologies, light transmission and UV consistency are necessities. To determine a material's light transmission, light is directed through the material at a range of wavelengths and the percentage of light that passes through the piece is measured.
|
Figure 4. A company making a neonatal CPAP device that requires biocompatible materials may choose an acrylic polymer that is bondable, tough, and easy to process. |
Light transmission is critical for microfluidic lab-on-a-chip applications. Although not typically used in the home, such applications are an example of the fast-growing trend of small devices developed in response to rising healthcare costs. The market for these devices, which allow doctors to perform tests on-site, is expected to grow from $84.3 million in 2005 to $200.4 million in 2012.4 These devices facilitate immediate diagnoses, resulting in a faster patient-care cycle and possibly lower treatment costs.
Ability to transmit light is critical for these devices because, in lab-on-a-chip analysis, an optical measurement of a treated sample at a specified wavelength is taken. The more light that is able to pass through the device, the better the measurement capability. For example, compare a material with 65% light transmission at 340 nm and a material with 25% light transmission. As a percentage of the value being measured, the material transmitting less light has a much greater relative error and a higher risk of inaccurate analysis.
In addition to light transmission, lab-on-a-chip devices require materials with other properties for accurate sample analysis. Transparency and optical purity are important to minimize fluorescent interference with the measurement response. The material's UV consistency is also critical for the optical windows through which the measurements are taken. Finally, the material used to mold the microchannels in these devices must have dimensional stability for controlled fluid flow.
Conclusion
There is no end in sight for growing healthcare costs. But enhancing process efficiency and adapting production processes to meet the demand for home-care devices are two strategies that medical device OEMs can use to stay competitive. By leveraging the characteristics of acrylics and other advanced plastics, manufacturers can realize cost savings through improved manufacturability. However, OEMs must continue to stay vigilant when choosing materials for their products. It is important that they carefully consider their devices' specific requirements and that they take advantage of their vendors' expertise when making their materials selections.
Peter Colburn is technical manager, molding compounds, at Cyro Industries (Parsippany, NJ), a subsidiary of Degussa Corp. He can be reached at [email protected]. Holger Tinz is market specialist, medical segment, at Cyro. E-mail him at [email protected].
References
1. David R Francis, “Why the Healthcare Crisis Won't Go Away,” Christian Science Monitor, July 18, 2005.
2. “Technology and the Rising Cost of Health Care” [online] (Chicago: Blue Cross Blue Shield, 2004); available from Internet: www.fepblue.org/toyourhealth/tyhhctechnologycosts.html.
3. Alpesh Gandhi, “Market Input: The Home-Healthcare Market” (San Francisco: Frost & Sullivan, 2005).
4. “U.S. Microfluidics/Lab-on-a-Chip Markets” (San Francisco: Frost & Sullivan, 2006).
Copyright ©2007 Medical Device & Diagnostic Industry
About the Author(s)
You May Also Like