Transforming Medical Practice
A number of MDEA winners that use design principles and technological advances to offer therapeutics or diagnostics just may change the future of certain treatments.
April 1, 2007
MDEA 2007
Quite a few of the 2007 Medical Design Excellence Award winners have the potential to transform—or disrupt, to use an economics term—certain standards of care or the way specific procedures are performed. These transformative technologies do not necessarily represent the most technically innovative or the most complex of the winners. Rather, they have found ways to make users rethink the way a task could be done.
“Radical innovation—particularly where it may compromise simplicity and safety—was not necessarily a characteristic of the MDEA winners,” says MDEA juror Ogan Gurel, MD. “[But] innovation is alive and well in the medical device industry…the safety element integrates the concepts of aesthetic design, simple execution, and intelligent process.” Gurel is chairman of Aesis Research Group.
As another example, says juror Michael Wiklund, president of Wiklund Research & Design, is in miniaturization. “Medical devices continue to get smaller and smarter by virtue of component miniaturization and the low cost of computer displays,” he says. “This means laboratory capabilities are now provided in the form of rolling workstations, and workstation capabilities are now provided in handheld devices.”
Here are some examples of how outstanding medical device design is changing the practice of medicine.
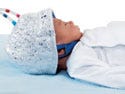
Cool-Cap Neonatal Head-Cooling System
|
Olympic Medical's Cool-Cap system is easy to set up, delivering the vital treatment in less than 15 minutes. |
FDA approves about 4000 medical devices per year. But it issues a press release about a new device approval only a handful of times a year. When it did so for the Cool-Cap in December 2006, that said a lot about the device's novelty and importance.
About three in every 1000 U.S. newborns suffer from moderate to severe hypoxic-ischemic encephalopathy (HIE), where conditions of low-oxygen supply to the brain can cause severe neurological injury or be fatal. There was no treatment for this until the Cool-Cap system came along. It is made by Olympic Medical Corp. (Seattle), a unit of Natus Medical Inc. (San Carlos, CA).
The Cool-Cap system lowers the temperature of the brain (without significantly lowering the temperature of the body) to prevent or sharply reduce the severity of neurological injury associated with HIE. It has three main components. A cap fitted atop the baby's head maintains hypothermia by means of the cooling water circulated through it. The cooling system consists of a thermostatically controlled water cooler that quietly and efficiently maintains the required temperature. An electric pump and plastic tubing system causes water to flow to the cap.
Work on the system began back in 1997, after the editor of the journal Pediatrics cast light on hypothermia in newborns. Researchers in London and in Auckland, New Zealand, were experimenting with methods to treat it, but they needed funding to develop a piece of equipment and to run clinical trials. Olympic, which makes cerebral function monitors (CFMs) that can detect HIE, stepped in, providing not only corporate funds but also personal funds for this important project. Olympic's first unit was delivered to Auckland in 1998, and clinical trials began in 1999.
The cooling method envisioned by the researchers worked, and Olympic's next step was to make a commercial version of the device. Its hardware is similar to that of the firm's other CFMs, which hospital personnel were already used to, says Ted Weiler, PhD, Olympic's vice president and R&D director.
|
The treatment works best when administered within the first six hours of birth, he says. Therefore, the system had to be easy to set up, otherwise essential time would be wasted. So Olympic designed an interface that walks the user through each phase of the setup, enabling treatment to occur in less than 15 minutes.
FDA reviewers took a major interest in the product when they became aware of it. But rather than cutting corners to fast-track it, they applied extra rigor to the review, because they felt the application was so important that the technology had to be done the right way, Weiler says.
“The panel asked for three things. One was [for us] to train our customers and another was to set up a registry. Those were easy,” Weiler says. “But then they wanted to make sure that we followed the exact protocol that was used in the clinical trials. They wanted to be sure that it was the same in a chaotic environment as it was in a controlled environment. That meant we must use the CFM—and specifically, the Olympic CFM device that we used in the trial—to determine which infants would need the Cool-Cap. We built the protocol into the system using setup wizards and a very user-friendly graphical user interface.”
Weiler says there was a partnership between the agency and the firm during the review process. “There had to be: This was a cure for an incurable disease.”
“This was one of the best devices we saw,” says juror Gail Baura, PhD, a professor at the Keck Graduate Institute. “It is part of a trend of medical devices being developed to solve very specific needs.” The device won an award in the category of Critical-Care and Emergency Medicine Devices.
Halo360 Esophageal Tissue Ablation System
|
The Halo360 system by Bârrx can reliably ablate tissue afflicted with Barrett's esophagus using an electrode array. |
The diagnosis of esophageal cancer is all too often a death sentence for sufferers. Doctors can spot a condition called Barrett's esophagus, a precancerous state of esophageal tissue. But there had not been a reliable way to remove tissue afflicted with Barrett's until the advent of the Halo360 system, developed by Stellartech Research Corp. and manufactured by Bârrx Medical Inc. (both in Sunnyvale, CA).
The devices that make up the system work together to ablate the esophagus while controlling the depth of the ablation. If too shallow, harmful tissue is left behind; if too deep, good tissue is ablated, which can be problematic.
A sizing balloon is inserted to measure the internal diameter of the esophagus so that the appropriately sized ablation catheter may be selected. The ablation catheter is a balloon catheter mounted with a closely spaced bipolar electrode array. The array circumferentially ablates a 3-cm segment of Barrett's epithelium in less than 1 second. The close spacing of the array achieves a uniform superficial depth of ablation (<1000 µm). An energy generator inflates and deflates the sizing balloon and ablation catheter, automates the sizing procedure, sets the ablation parameters to optimize the energy density, and supplies energy to the ablation catheter.
The idea of using some sort of energy to ablate Barrett's esophagus had been around for a while, but no one had figured out how to provide a device whose effects are uniform and precisely limited in depth. The device treats a relatively large surface area in a single radio-frequency (RF) energy delivery cycle and is safe and cost-effective, says Mark Colella, Bârrx's director of marketing.
“The esophagus is different in every patient,” he says. “That meant we had to match our technology to different patient anatomies.” Doing so was extremely important because the depth of the ablation had to be uniform. Therefore, the company discovered the need for the sizing balloon.
The catheter removes all or nearly all of the Barrett's tissue within a 3-cm region. If the area to be ablated is larger than 3 cm, the physician advances the catheter another 3 cm and repeats the treatment. In trials, 70% of patients who had the Halo treatment had no signs of Barrett's esophagus after one year. The other 30% were 90–99% free of it, but had small spots that didn't show up on biopsy. These were usually hidden in tissue folds. For those patients, Bârrx has unveiled the Halo90 system, a smaller device that ablates any spots missed by the Halo360, says Colella.
“I was impressed with the reported medical efficacy of this device, essentially curing a precancerous condition by means of a comparatively fast and simple tissue-ablation procedure,” says Wiklund.
Juror Mary Beth Privitera says she appreciated that “unique manufacturing techniques were developed to produce the copper-based point of the energy delivery area.” She also liked that “seemingly small details, such as a display to walk the user through the procedure, make this system easy to use. In my opinion, this represents the trend to design beyond the critical ablation device, or point of care, to consider the steps required in delivering that care.” Privitera is codeveloper and faculty member of the University of Cincinnati's Medical Device Innovation and Entrepreneurship Program.
Bârrx is a spin-off company from Stellartech, which develops technologies and then forms new companies to commercialize them. The Halo system won an award in the Radiological and Electromechanical Devices category.
Mammotome MR Breast Biopsy System
|
Ethicon's Mammotome is compatible with MRI, enabling detection of lesions in the breast that was not previously possible. |
Breast biopsy systems have been around for a long time, used in conjunction with x-ray or ultrasound systems for cancer detection. But certain kinds of lesions in the breast are not detectable with those technologies. Many of them are detectable with magnetic resonance imaging (MRI). Unfortunately, vacuum-assisted biopsy systems are not compatible with MR technology. The Mammotome, developed by Ethicon Endo-Surgery (Blue Ash, OH), rectifies that problem.
MR can identify lesions in dense breast tissue, making it the preferred screening method for young women or those genetically prone to breast cancer. ��“The market trend is to use MR, so we had to come up with something compatible,” says Dan Haberstich, staff industrial designer for Ethicon Endo-Surgery, a Johnson & Johnson company. “It couldn't wait.”
The problem was that the strong magnetic field generated by MR means that metal objects can be drawn to it, and even objects that are safe to use with MR may come out distorted on its images. Also, the coils that position the breast to be imaged left little space for a biopsy needle to be attached. And traditional MRI units have barely enough room for a patient, much less room for a biopsy device too.
The solution was to design four subsystems. First, a combination drive unit and power cable was developed to allow the MR-unsafe control unit to remain outside the magnet room. Next, an accurate, stable localization fixture provided an intuitive method to attach and orient the biopsy device on the breast coil. Third, a disposable targeting set was attached to this fixture. It was able to enter the tight space in the MR magnet and be visible under MRI without distortion. Finally, a disposable probe was mounted to the localization fixture to interface with the targeting set and place the cutter in the targeted tissue. Once attached, it can be operated hands-free, minimizing inadvertent movement.
While existing technologies have access to less than half of the breast tissue, the Mammotome is designed to be able to access 70% of it. That was achieved by, among other things, devising a new breastplate design that takes up less space and allows access to certain areas. That design, in conjunction with the new localization fixture, enables clinicians to target lesions within 2 mm, compared with the previous standard of 6 mm. The system also offers multiple targeting and probe lengths, giving the operator more options and increasing patient safety by allowing for shallower penetration depths in certain situations.
In the end, Ethicon Endo-Surgery came up with a better way to do minimally invasive breast biopsies. “It makes for less of a scar and quicker healing, while identifying lesions more effectively,” says Haberstich. That helped the system win an award in the category of Surgical Equipment, Instruments, and Supplies.
Juror Edmond Israelski, PhD, says the system offers “a great set of tools to allow more-efficient and accurate needle breast biopsies to be taken. It also has an elegant graphical user interface to more easily guide the physician in collecting biopsy tissue. This MRI-compatible system will benefit many patients by allowing considerably more less-invasive biopsies to be performed.” Israelski serves as human factors program manager for Abbott Laboratories.
Piccolo Xpress Chemistry Analyzer
|
The Piccolo Xpress Chemistry Analyzer by Abaxis enables healthcare providers to see 14 different blood chemistry values from just 100 µl of blood. |
The first generation of the Piccolo Xpress, made by Abaxis Inc. (Union City, CA), was the first compact clinical system designed for near-patient testing. It could produce results that used to take 48 hours in only 15 minutes or less. The new version, a winner in the In Vitro Diagnostics category, makes the process even simpler.
“The user interface was completely redesigned so all you have to do is load the sample, push a button, and get results,” says Kenneth Aron, PhD, vice president of research and development for Abaxis. “There is no preanalysis treatment. You can run it with whole blood, serum, or plasma.” The system allows providers to perform routine multichemistry profiles using just 100 µl of blood, getting up to 14 different blood chemistry values. Its design is vertical and compact, saving valuable space. It is so simple to use that an untrained operator can do it. And it is flexible enough to be used in doctors' offices, hospital rooms, surgical suites, or battlefields.
The trick to such functionality is in the analyzer's centrifuge-like action. It has a sophisticated motion controller capable of generating great velocities, a microprocessor with extensive computing capability, and precision optical interference filters. It also offers high-performance op-amps and large-area UV-enhanced photodiode detectors.
It was not an easy process to develop the new version, says Aron. “We had to increase throughput and decrease run time,” he says. “We wanted to get CLIA waived for many applications, which we are. The instructions for use had to be unmistakable. We needed the processing power that had been developed over the last 10 years. It took 18 very rigorous months.” For the industrial design, Abaxis enlisted French designer James Toleman, who made many of its ideas a reality.
Indeed, for FDA to allow untrained operators to use such a device, the agency had to be convinced of its simplicity. It was, and the Piccolo became one of the few medical instruments on the U.S. market to have such approval. Buttons were eliminated in favor of a touch screen, a format that any user would find familiar. Abaxis used flow diagram charts to come up with a simple menu structure and graphic design. And its design allowed the results to be easily displayed and printed on adhesive labels for use with patient charts.
How was this accomplished? By attentiveness to customer needs. “We had been working very closely with our customers for a long time,” says Aron. “We had been collecting information about how they used the instrument and what its strengths and weaknesses were. We took that information and applied it to the specifications of the new product.”
Jurors liked the design features and usability of the analyzer. “The size and portability of the analyzer is an advantage over other systems, but most impressive is the simplicity of use,” says Craig Jackson, PhD, president of Hemosaga Diagnostics. “The ability to operate the instrument from a 12-V-dc source, something that might be necessary in circumstances in which power failures occur, is an asset.”
“I was impressed with the small size of the disposable reagent rotor—smaller than a hockey puck—that contained wells for more than a dozen factory-calibrated reagents, and required only a 0.1-cm3 blood sample per panel,” says Robert Virag, founder and principal of Trifid Medical Group and Alveoli Medical.
UltraCrit Hematocrit Measurement Device
|
The UltraCrit by Separation Technology uses low-energy ultrasound to measure hematocrit levels. |
Measuring hematocrit levels in human whole blood used to be a job for a large, expensive, and sometimes slow device. Then Separation Technology Inc. (Altamonte Springs, FL) came up with the UltraCrit, which is the opposite of that in almost every way.
The key to the transformation of the technology was the use of ultrasound, which allowed for major advances in precision and speed. Ultrasound had never been used to try to distinguish characteristics of blood, so the firm had quite a task ahead. The development process took years, but it paid off with an award in the In Vitro Diagnostics category.
“The use of low-energy ultrasound to quickly determine the hematocrit is a real innovation,” says juror Jerry McVicker, PhD. “The speed and accuracy of this device will change the way this test is performed. In addition, the overall design is outstanding.” McVicker is scientific director of Midland Bioproducts.
The initial tests were conducted with large blood samples (more than a quart of blood), using a range of transducers. Test times ranged from several minutes to much longer. Over the course of several years, several thousand hours of testing, and many design iterations, the technology evolved to use less than one drop of blood, a custom miniaturized transducer, and a measurement process that takes 30 seconds or less. New algorithms, processing techniques, blood collection, and handling methods were developed, as well as new hardware and software. This was an incremental process that paid particular attention to how the users would like to handle the blood sample and interface with the technology. At the end, the firm had a technology that was incredibly precise and repeatable.
A one-time-use cuvette is used to collect a single drop of blood via capillary action, usually from a finger stick, which is then loaded into the device. Once loaded, the device automatically starts the measurement process, which takes approximately 30 seconds. Yet, the firm's data indicate that the UltraCrit's repeatability and accuracy is comparable to large lab-based analyzers, despite being a fraction of the cost. (Hematocrit is a relative volumetric measure of red blood cells, and thus an indicator of general health. FDA requires that blood donors have a level of 38 or higher.)
The device is simple enough for any level of operator to use. It has screen-prompted operating instructions for use in CLIA-waived environments by entry-level medical technicians performing the blood screening test. The firm developed the device after consulting blood-bank technicians about what they like and don't like about screening tests.
“I found this product to possess the same level of design appeal as the better handheld consumer electronic products,” says Wiklund. “Instead of resembling a technical lab instrument, it looks and feels nice while also performing its primary blood analysis task. It matches the pattern of glucose meters that are designed more like consumer products than scientific instruments, so they compare favorably with the user's cellular phone rather than an engineer's multimeter.”
Vicks Forehead Thermometer
|
Kaz's Vicks Forehead thermometer uses the temporal artery to survey temperature. It is intuitive and easy to use. |
A new thermometer may not seem like a transformative technology. But consider this: oral, ear, and rectal thermometers are in some way invasive. A forehead thermometer is not. Yet the forehead thermometer returns results much more quickly.
“It uses the temporal artery and surveys the temperature under your skin,” says Janet Vilano, manager of innovation and design for its manufacturer, Kaz Inc. (New York City). “When you sweep it over your forehead, it's covering the area through which the artery flows.”
To get the reading, the Vicks thermometer uses infrared technology, the same as found in ear thermometers, but it is adjusted to scan the temporal artery.
The technology is ideally suited for use in children because of its noninvasiveness. But Kaz took other measures to increase comfort and ease of use.
“Because the thermometer was being developed for use on infants and children, thermoplastic rubber (TPR) was used around the sensor, making this area extra-gentle when applied to the skin,” says Vilano. “TPR on the probe also provides a materials-driven way of keeping the probe cover in place when the device is not in use. The gasket around the perimeter of the product offers a tactile area for improved grip, and it again protects the integrated memory button on the side of the lens area. The polycarbonate lens resists scratches and protects the tri-color backlit liquid-crystal display (LCD).”
The LCD itself was designed with the user in mind. “The thermometer has a large, color-changing backlit display that helps take the guesswork out of interpreting a temperature by offering a color—green, yellow, or red—that corresponds with the measurement,” says Vilano. “The design team felt that the ideas of ‘normal,' ‘elevated,' and ‘fever' could be represented in colors to further help the user interpret results immediately.” Interviews with parents and kids and experiments with mockups contributed to the final design. This careful consideration to usability helped the product win an award in the Over-the-Counter and Self-Care Products category.
“This is a new paradigm in taking temperature noninvasively,” says juror Pascal Malassigné, professor of industrial design at the Milwaukee Institute of Art and Design. “It takes the stigma out of having your temperature taken.”
“This product is very intuitive. Moms and dads all over the world place their hands on their children's foreheads to determine the presence of fever,” says jury chair Herbert Voigt, PhD, president of the American Institute of Medical and Biological Engineering. “The Vicks Forehead thermometer is like an extension of the hand.”
Conclusion
Indeed, intuitiveness, usability, and an outside-the-box approach to design are how a medical device that is as simple as a thermometer can find common ground with a device that is as complex as a brain-cooling system.
Copyright ©2007 Medical Device & Diagnostic Industry
About the Author(s)
You May Also Like







.png?width=300&auto=webp&quality=80&disable=upscale)
