Survival of the Smartest
As medical device technology evolves and manufacturers create products that do more with less, the industry has seen an influx of devices with sophisticated data and software capabilities. These “smart” devices with enhanced features can range from diagnostic equipment to monitoring devices to pill bottles. They often rely on embedded software to store, sort, and analyze data as well as add functionality.
April 1, 2010
Just as in other industries, such as consumer electronics, software is what often delivers functionality in the medical device space. For example, functions that were performed with knobs and buttons are gradually being performed via a touch screen display. In many cases, the data gathered by software in these devices are then whisked away, typically via a wireless technology such as Bluetooth, to the user’s PC or to a care provider to be organized and analyzed. What was common among many of the devices that won Medical Design Excellence Awards in 2010 was not necessarily game-changing design, but rather clever incremental changes—which can be just as innovative.
Hi! It’s Your Prescription Calling
|
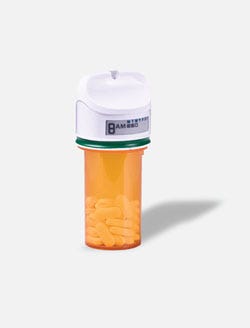
The GlowCap notifies the pharmacy when a prescription needs refilling. |
Whether elderly or young, people aren’t always diligent about taking prescription medication at the instructed time or frequency. According to a 2009 New England Healthcare Institute study, nonadherence to prescription medication is costly to the U.S. healthcare system—to the tune of nearly $300 billion annually. Pill takers may not need to be harassed about adherence, but a new device can make sure they are at least gently nudged.
The GlowCap, manufactured by Cambridge, MA–based Vitality Inc., fits on standard pill bottles and sets itself apart by having intelligence embedded in the cap. Noncompliance is addressed by using both light and sound to signal medication time. A reminder light, the Health Hub nightlight, comes with the cap, which wirelessly transmits adherence data to the light using a local 915-MHz radio frequency. (The light then sends data to Vitality using a cellular connection.) The plug-in light flashes when it’s time to take medication. Then the light plays an arpeggio similar to a cell phone ringtone. If these signals are ignored for two hours, the patient receives a reminder phone call: “It’s time to take the pill in your green GlowCap,” the automated voice might say.
“These reminders are seamlessly integrated through wireless communication, which provides feedback to the system as to whether medication has been taken. It is a great example of taking common technologies and combining them in a synergistic way for a needed application,” notes Gail Baura, an MDEA juror and professor at the Keck Graduate Institute of Applied Life Sciences (Claremont, CA).
The device also makes it easier for physicians and family members to monitor noncompliant patients—it records when a pill bottle is opened and compiles monthly progress reports for patients that can be sent to their doctors. The user can also opt for weekly e-mail reports for themselves, family, or care providers. The device also takes some of the hassle out of getting refills by notifying the pharmacy when the pill supply is dwindling.
Vitality Inc. CEO David Rose is no stranger to the technology embedded in the cap. His previous company, Ambient Devices, made wireless information indicators, such as energy price indicators (think of an orb that changes colors based on the price of energy, which gives people the chance to curtail their home energy use when it is too high). “What we found was that if you have a device in peoples’ homes that’s always on and in their peripheral vision, it serves as a constant reminder and also a sort of persuasive device,” Rose says.
The GlowCap was also borne from Rose’s personal experience. Many of his older relatives died of heart disease, and he knew that he was genetically predisposed. “My father had bothered me to take an aspirin everyday to try to reduce my risk and I was always challenged to do it. I realized that the best way to motivate myself was to create a feedback loop where he would know if I took my meds, and I would know if he took his meds.”
In the end, Vitality managed to innovate while still using a plain pill bottle.
“By selecting an ordinary amber pill vial as the vessel and just reinventing the cap, we were able to create a device that wouldn’t change the way pharmacies and pharmacists do their work,” Rose says. “One innovation was [to be] fairly incremental about it.”
Do You Hear What I Hear?
|
Detecting heart murmurs has been made easier by software integrated into the Littman stethoscope. |
Sometimes innovation is simply taking something pedestrian and making it novel again, which is the case with the Littmann electronic stethoscope Model 3200 manufactured by 3M (St. Paul, MN). This fourth-generation device is brainier than traditional stethoscopes thanks to built-in Bluetooth and software components. 3M has partnered with Zargis Medical Corp. (Stamford, CT) for the various iterations of the device, using Zargis’s Cardioscan heart sound analysis software to make the stethoscope’s features more robust.
“The digital Littmann stethoscope represents in many ways an important milestone at the intersection of information technology (e.g., Bluetooth), software-based analysis of heart sounds, and diagnostic devices in healthcare,” says juror Balakrishna Haridas, president of Device & Implant Innovations LLC (Mason, OH). “The device has been designed to emulate the current well-known stethoscope and its form factor with a twist—in that it allows a physician to record and analyze the acoustic signatures heard during patient examination.”
One school of thought believes that today’s clinicians are not as skilled as their predecessors at auscultation, the process of listening to the body’s internal sounds. Overreliance on advanced technology, e.g., echocardiograms, is cited as the main reason for this trend. But as Zargis’s chief medical and technology officer Alan Stein explains, auscultation can be a bit more complicated than it sounds.
“The first and second [heart]beat are easy to distinguish, but when a murmur presents itself, it’s sometimes right on top of those beats, and that’s what makes it challenging,” he says. “Or you could have a patient who doesn’t want to disrobe and now you have to listen through clothing, or you have an obese patient and the sound doesn’t travel as well through the body. The layperson doesn’t understand how hard it is to auscultate.”
As a result, physicians are often unsure whether a patient should be referred to a cardiologist, which can add a host of unnecessary costs if the patient is fine. Zargis and 3M set out to make it easier by amplifying the internal sounds that clinicians are trying to listen to and reducing ambient noise. A digital signal processor manages the incoming acoustic signal, then a speaker in the stethoscope converts the digital signal into an audio response. A Bluetooth data link sends the digital signal to a PC for visualization and further analysis. It also enables communication with the Cardioscan software, which can be installed on the physician’s computer. The enhancement of sounds translates into an enhancement of the clinician’s ability to diagnose cardiac, pulmonary, and other internal pathologies.
“Our position was to give the primary care physician more information about the heart murmur that they may hear—to help them better make a decision on whether it should be referred on [to a cardiologist],” says Tim Chismar, technical service engineer at 3M. “Information technology has increased so dramatically over the last 20 years…it will be the future of medicine to apply algorithms of this sort to give the healthcare provider more information and to help them make better decisions.”
The algorithms to enhance sound, which Stein says have been in development for about a decade, are key to the device. Clinicians weren’t eager to adopt early electronic stethoscopes because even though they might have amplified sound, they amplified every sound.
“So it doesn’t help your ability to pick out the intricacies of sounds,” Chismar says. “Integrating a Bluetooth chip is one thing, but more challenging is perfecting the sound quality.”
Flash Drive, Meet Glucose Monitor
|
Consumer electronic devices were the inspiration for the Contour, which uses a USB to plug directly into computers. |
The Contour USB blood glucose monitor from Bayer HealthCare LLC (Tarrytown, NY) introduces another example of incremental-but-clever change. On one end is the testing apparatus, and on the other is a USB. Once the latter end is plugged into a computer, diabetes management software automatically launches. The company touts the device as the first blood glucose monitor to plug directly into a computer, a factor that made MDEA jurors see the device’s utility.
“A major hurdle for people managing diabetes is interpreting and translating blood glucose readings into actionable steps that lead to healthier outcomes,” says Jim Best, a juror and founding partner of design consulting firm Pensa (Brooklyn, NY). “What sets the Bayer USB meter apart is its thoughtful application of current technologies, intuitive user interface, and onboard blood glucose management software that helps patients streamline this process, making this meter extremely easy to use.”
Other factors were equally compelling. “I liked the light weight, the contour of the machine, and the way the digital information read—it was easy,” says juror Denise Korniewicz, a professor and senior associate dean for research at the University of Miami School of Nursing and Health Studies. “The ability to upload immediately to a software package makes it easy to use and easy to send data to healthcare providers.”
Tracking blood glucose data and grasping their trends are key aspects of managing diabetes. The problem with some recent traditional blood glucose meters, according to Bayer, is that their user interfaces are complicated. As a result, many meter users track their data in a logbook—if at all.
“Patients are driving healthcare innovation and are key to disease management. The idea for the Contour USB meter was born out of the desire to marry the needs and insights of people with diabetes with technology,” says Staci Gouveia, deputy director of global communications at Bayer.
The Contour uses a five-second test time to simplify testing procedures. After the user applies blood to the strip, the glucose meter prompts the user to indicate whether the reading is before or after a meal. When the Contour is connected to a computer, preloaded software displays pre- and postmeal trends to help diabetics and their doctors get a clearer picture of blood sugar information.
“Mitigating usability [inconveniences] is very important. It creates the opportunity to improve compliance by allowing better access to features and functionality that help healthcare providers and patients successfully collaborate to manage the disease,” Best says.
According to the company, the meter’s aesthetic design was inspired by consumer electronics—primarily because users’ expectations are set by all the other portable devices they carry with them, such as cell phones and MP3 players. In another effort to cater to active users, the Contour uses a rechargeable battery. It has the power capacity to conduct 150 tests before needing recharging, which takes two hours.
The Compas Leads the Way
|
The Compas uses Bluetooth to communicate with computers and send gait data. |
Seven million data points sounds like a lot of information to gather. But Orthocare Innovations (Oklahoma City, OK), which manufactured the Computerized Prosthesis Alignment System (Compas), knew it would take work to remove the guesswork from lower-extremity prosthesis fittings. The company obtained those millions of data points by working with dozens of amputees, who helped perfect the technology simply by walking with different alignment settings.
Prosthesis misalignment has been linked to skin breakdown from uneven socket pressure distribution, joint pain from unbalanced loading of the knee or hip, and lower back pain. To ensure proper alignment, some prosthetists turn to gait laboratories. Gait labs offer computerized gait analysis, but that process can be both costly and time-consuming, not to mention that the data are usually best left to be interpreted by specialized technicians. As a result, this method is less helpful during therapy and rehabilitation.
“The Compas takes the trial and error out of the alignment process to deliver a perfectly fitted prosthesis,” says juror Pascal Malassigne, an industrial design professor at the Milwaukee Institute of Art and Design.
The Compas includes a titanium sensor that measures torques and determines the weight, balance, and gait timing of the prosthesis. A master unit is mated to the sensor, which is a permanent part of the prosthesis (Orthocare’s circuitry and sensor technologies are built into the prosthetic limb). The master unit provides the power and Bluetooth connection. The device communicates via Bluetooth with a graphical software system on the prosthetist’s computer, which automatically interprets the gait data with proprietary algorithms, then displays the data on screen. The software provides the prosthetist with direct, simple feedback for improving alignment.
“The Compas provides healthcare providers with a relatively simple, fast, and easy-to-use tool that can significantly improve the quality, comfort, and usefulness of lower limb prosthetics,” observes Richard Meyst, juror and founder of consulting firm Fallbrook Engineering Inc. (Escondido, CA). “Using actual data collected from patients, the prosthetist can precisely and accurately make alignment adjustments to the prosthesis which provide the patient with an efficient, comfortable normal walking gait.”
A target population for this device is veterans from the war in Iraq. As the combat enters its eighth year, the number of amputees has steadily increased. Orthocare introduced the technology to the U.S. Department of Veterans Affairs during the production phase specifically to reach this population.
“[The jurors] liked the fact that this product will help many of our returning armed service people who have been permanently impacted by distant wars. It is an excellent product with superior design,” Meyst says.
Conclusion
“One trend we’re seeing is more incremental software-based features,” says Yadin David, a juror and founder of Biomedical Engineering Consultants LLC (Houston). Indeed, that trend served many of the MDEA entrants well. They were able to take ordinary devices that the medical world has seen millions of times—pill bottles, glucose monitors, prostheses, and stethoscopes—and present them in a different light. Software and data features were key to the innovations.
Lawrence Lloyd is associate editor of MD+DI.
About the Author(s)
You May Also Like




.png?width=300&auto=webp&quality=80&disable=upscale)
