November 1, 2007
NEWS TRENDS
|
Five orthopedics companies settled with the U.S. Department of Justice in September over allegations that they may have paid kickbacks to surgeons in return for favoring the use of their hip and knee implants.
The five are Smith & Nephew plc (London); Stryker Corp. (Kalamazoo, MI); and Biomet Inc., Johnson & Johnson's DePuy Orthopaedics, and Zimmer Holdings Inc. (all Warsaw, IN).
Four of the firms, excluding Stryker, will pay a combined $310 million to settle the claims. All five will pay to have a federal monitor oversee their practices for 18 months. All but Stryker must submit to additional monitoring by the Department of Health and Human Services' Office of Inspector General (OIG) for five years. And all agreed to be bound by the AdvaMed Code of Ethics.
According to the information released by the government, all five firms showed strong cooperation once they were made aware of the investigation.
It was not made clear whether the questionable arrangements occurred after AdvaMed introduced its code of ethics. The government subpoenaed documents from January 2002 to March 2005.
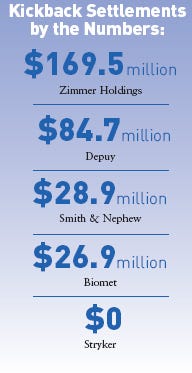
The code has prompted many device companies to institute stricter policies regarding interactions with caregivers. If the code didn't provide enough incentive for firms to prohibit kickbacks, the settlements might. The settlement amounts are as follows:
Zimmer, $169.5 million.
DePuy, $84.7 million.
Smith & Nephew, $28.9 million.
Biomet, $26.9 million.
Stryker, $0—but the government reserves the right to seek civil damages should the firm not comply with its agreement.
According to the government, surgeons accepted lavish gifts, vacations, and “consulting fees” as high as $200,000 from the firms in return for recommending their products. The government got involved because it decided the practices constituted a defrauding of Medicare. The five firms control nearly 95% of the U.S. market for reconstructive implants.
Stryker was spared a fine and the OIG monitoring requirement because it was the first to cooperate. All firms except Stryker had criminal complaints filed against them, but each of the four signed a “deferred prosecution agreement” that will allow for the criminal complaints to be dropped should they reform their practices to the government's satisfaction. Stryker signed a “nonprosecution agreement.” Each of these agreements specifies in great detail what sort of business practices the firms can and cannot engage in.
The government did not identify or charge any surgeons, nor disclose how exactly the schemes worked.
An unidentified attorney from one settling firm told the Star-Ledger of Newark, NJ, that the other firms were shocked that Stryker got no fine, because the firms believed that all were receiving the same deal. He speculated that the public could come to believe that Stryker did nothing wrong.
The text of the complaints and the agreements can be viewed at www.usdoj.gov/usao/nj/press/2007releases.html.
Copyright ©2007 Medical Device & Diagnostic Industry
About the Author(s)
You May Also Like