Opening the Doors to India: Offshoring Medical Devices
Although challenges face device firms that offshore in India, the country offers numerous opportunities for firms that do it right.
March 1, 2007
GUIDE TO OUTSOURCING
|
India's workforce is well educated and qualified. Therefore, many OEMs consider India to be a good outsourcing destination. |
The U.S. market for medical devices is expected to reach $89 billion in 2007, representing a promising opportunity for manufacturers and service providers.1 But with this opportunity comes the added pressure for medical device companies to stay ahead of the competition. To gain a competitive edge, medical device companies are faced with the challenge of developing new, innovative products. Alternatively, they can create new versions of existing products that adopt the latest methods of treatment and conform to the highest quality standards. Either way, firms are pushed to accomplish their goals at a reduced cost.
To compete in this attractive market, many medical device makers are successfully partnering with offshore outsourcing firms and collaborating on device development and manufacturing. They are offshoring processes such as application development, systems engineering, hardware design, software solutions, and manufacturing. This way, U.S. OEMs can focus on more-strategic, value-added projects and divert substantial funds to R&D.
The benefits of partnering with an overseas outsourcing company are substantial. However, there are also risks and obstacles that should be assessed carefully. Beyond the cultural differences and communication barriers, firms should also consider economic stability, fluctuating currencies, trade barriers, regulatory processes, and standardizations, to name a few.
Benefits of Outsourcing to India
Many consider India to be an excellent outsourcing destination. With more than 380 universities, 11,200 colleges, and 1500 research institutions, India has the second-largest pool of scientists and engineers in the world. More than 2.5 million graduates are added to the workforce every year, including 300,000 engineers and 150,000 information technology (IT) professionals. India's workforce is also one of the world's youngest. Among Brazil, Russia, India, and China, India is expected to stay the youngest. Its working-age population is estimated to represent 70% of the total population by 2030—the largest in the world. By then, the country is expected to have an additional 200 million people entering the job market.
It is because of this impressive pool of well-educated, highly qualified, English-speaking professionals that many experts consider India a good outsourcing destination for manufacturers and service companies. India offers a mix of engineering expertise, comparatively lower wages than those in the United States, high-quality product development, and proven experience with long-distance project execution. Furthermore, the United Nations predicts India will overtake China to become the most populated country in the world by 2035. This forecast indicates the prospect of continued growth is promising, to say the least.
India's economic and political stability have also contributed to its rise as an outsourcing destination. With a fast-growing economy, robust financial system, and stable political system, companies can reduce the risk of external economic or political issues affecting their business. Being a democracy ensures a stable policy environment, and India's independent institutions guarantee the rule of law.
In the medical device industry, products introduced or upgraded within the last five years typically drive a company's profits. Patent protections and the long cycle of clinical trials are key factors for newly released products. In addition, public acceptance is necessary for achieving a return on investment (ROI). And although the task of collaborative product design and manufacturing may seem daunting at the outset, the benefits can greatly outweigh the initial hurdles.
Leveraging the services of an Indian offshore partner that specializes in finished-device manufacturing is often a good choice. Such partners can help a company improve quality while shortening the product development cycle and reducing time-to-market. In addition, it can decrease costs by as much as 60%, according to HCL estimates. Although some may worry that lowered labor costs could result in longer development cycles, for many of the established and experienced offshore companies in India, this is not actually the case. Many of these companies have years of experience working at this capacity. Therefore, robust offshore development processes have been created, tested, retested, and fine-tuned to reduce costs and shorten development cycles.
Some Indian companies have expanded their manufacturing capabilities by developing an infrastructure and adopting manufacturing processes that uphold the strictest of standards. And some have added clinical trial and FDA regulatory certification processes to their portfolios. Finally, with the implementation of a high-quality telecom and connectivity infrastructure in India, it has become convenient for these companies to provide maintenance and field support as well.
Risks of Offshoring to India
Although there are benefits in outsourcing to India, there are always risks that should be considered. When seeking an Indian outsourcing partner, it's important to be cautious. Many vendors are growing rapidly, which could compromise the quality of their services. Visiting the facility and meeting in person with the project teams before selecting a partner or vendor can give OEMs a better understanding of the vendor's capabilities and the resources that would be dedicated to their project. Regular communications and visits will help validate the vendors' skills.
Managing the Entire Life Cycle of a Medical Device
|
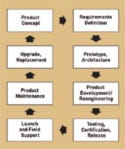
Figure 1. (click to enlarge) Life cycle of a medical device (clockwise from top). |
Indian outsourcers can help OEMs compete in the industry with quality products at a reduced cost, as they are able to manage the entire life cycle of a medical device (see Figure 1). The management should start from the product concept phase, when emerging and next-generation products are conceptualized. This phase brings together the skills of technology and the domain knowledge of physicians and research team. Then, the vendor in India should create a detailed requirements document in close collaboration with the OEM.
This document is often reviewed by the OEM's marketing department for market feasibility, as well as by user groups such as physicians, patients, and regulatory bodies. Once the requirement document is finalized, the outsourcing company in India can develop a prototype along with a detailed architecture. Once the architecture is approved, the India-based outsourcer develops and consults on the software development, mechanical design, and electrical considerations of the product, depending on the needs of the device.
Evaluating the Viability of an Offshoring Model
To determine whether offshoring is a viable option, certain critical factors should be carefully evaluated, including regulations, accountability, the total cost, and resource optimization. This industry is heavily regulated, so understanding the regulations that will affect the relationship is vital.
It is also advisable to obtain background information on potential offshore partners' experience and track record for compliance with specific regulations. A number of reputable research firms, such as Gartner and AMR Research, can provide information, research, and analysis about the companies that work in the device field and their respective processes. It can also be helpful to talk to companies that have already outsourced overseas. Many large medical device companies are already working with a number of Indian contract manufacturers. Speaking with those involved with the implementation and execution of an offshore project is an excellent way to gain insights into such collaborations.
In addition to the compliance and regulation challenges, OEMs need to ensure that a potential offshoring model allows them to achieve the benefits of offshoring without compromising the quality of their products. With an approach known as risk sharing, an OEM and an offshore partner use a model centered on output-based pricing. With output-based pricing, the OEM pays only for output or results. It examines factors including managing expectations, establishing communication processes for all levels, identifying appropriate contacts from both companies, establishing formal progress reviews on a regular basis, and striving for cultural synchronization. Many quality control problems can be managed, and timely deliverables can be achieved, by structuring the OEM-vendor relationship around these principles and fundamentals.
With offshoring, one of the most difficult areas to assess is the total cost of ownership. The traditional ROI equation is based on subtracting net costs from net benefits. However, because of the complexity of these relationships, it is important to conduct a careful analysis of the costs of outsourcing. Then compare those results with the costs of an in-house solution. The results can help shape a device OEM's strategy.
Another benefit of offshoring is resource optimization. Indian contract manufacturers can leverage certain resources as necessary during the progress of a project. For example, during the testing phase, additional resources can be brought in to perform the testing and then be reallocated once their work is complete. However, this flexibility can have both positive and negative effects. Mapping out plans for internal and external resources and understanding the value they offer to the model in both the short and long term is critical. Also essential is managing expectations with respect to the effect offshoring can have on an organization.
Choosing an Outsourcing Partner
However, certain steps can minimize the risk and help OEMs reap the benefits of offshoring in India.
Choose an FDA-registered partner with capabilities and experience across all classes of devices, as well as a proven track record. Device OEMs should also look for certifications, such as the medical quality management system (MQMS) certification, per ISO 13485:2003 and ISO 14971, which ensures that risk management standards are met. Companies should also employ an effective risk management program that includes software hazard analysis, failure mode effects analysis, and worst-case and reliability analysis.
Additionally, OEMs should investigate a prospective outsourcing partner's knowledge of FDA and the Medical Devices Directive. A potential partner's experience in designing and developing medical device products in a regulated environment should also be researched. Determining a vendor's flexibility and willingness to work in different models, such as a fixed-price base, is also advisable. Finally, OEMs should require a predictable and transparent delivery model. All of these questions will be best answered through in-person meetings and ongoing site visits.
Establishing an Effective Outsourcing Partnership
At the onset of any partnership with an outsourcer, the structure of the relationship should clearly define expectations, communications, contact persons, reviews, and cultural sensitization. Managing expectations is important from both the OEM and the vendor perspectives. Some firms will even start by conducting an on-site requirement study. Such studies usually encompass product definitions, functionalities, technology, cost of development, staff, and standards.
As in any partnership, clear communication is essential. It improves efficiencies and can eliminate errors owing to miscommunication. India has the talent pool of educated, English-speaking professionals, which helps ensure clear communication. However, despite the common language, there are still cultural differences that need to be understood from the start of the relationship. Different communication styles are the most common cultural issues that can cause problems or upset relationships between U.S. and Indian workers. For example, if someone in the United States says “yes” to a task, it is understood that that the task will be completed. However, in India, “yes” often means, “I want to do it, and I will do everything I can,” but it is not a promise. Understanding differences like these are key to managing expectations and growing a successful relationship.
Also, Indian companies place a very high value on face-to-face meetings. Therefore, as the teams are being established, many Indian outsourcers will encourage meetings in-person or via video conferencing. After the formal introductions, the two companies should establish protocols for transferring data and critical information. Both parties should create a communication mechanism among the key stakeholders in the executive team, operational teams, and the day-to-day operations team. Next, identify the right contact persons from both organizations. It is helpful to agree upon a robust review mechanism that establishes weekly, monthly, and quarterly meetings. These reviews help resolve any conflicts before they can become larger problems. And, as mentioned, Indian outsourcers will likely encourage some of these meetings to take place in person or via video conferencing.
Cultural synchronization is key to a successful relationship with an Indian outsourcer. Resolving common cultural differences and establishing an open line of communication is critical. To overcome the cultural differences, some companies conduct cultural sensitivity workshops in which participants learn about the differences between cultures, verbal and nonverbal differences, etc. For example, the western norm for shaking your head from side to side means “no,” whereas in India, the same gesture can mean “yes.”
Executing an Effective Pilot Program
There are certainly risks in outsourcing medical products and services overseas. However, OEMs that carefully evaluate the viability of offshoring and carry out an effective pilot program are poised to see the long-term benefits of offshoring. Although not unique to India, this is an important starting point for companies to consider. A pilot program, which is a small-scale project that can last anywhere from 36–100 months, can include verifications, validations, and high-level design. Identifying projects that have the potential for early success can be a smart strategy. That way, the OEM can use the best practices of the program and work at a suitable pace to expand the relationship by scope and size. Additionally, early success will help build confidence and offer both firms the ability to refine their strategy as needed.
Conclusion
Through years of experience, Indian outsourcing vendors have developed effective processes, procedures, and skills that enable U.S. OEMs to overcome the obstacles and challenges that exist in this highly competitive market. And with a large workforce of young, talented, English-speaking professionals, plus a stable economic and political environment, India is well positioned to continue expanding its work with OEMs through the entire life cycle of a company's products.
India has firms with years of design and manufacturing experience, the certifications required, and a talented pool of engineers, and this combination enables U.S. OEMs to reduce costs. Moreover, India has experience working with a range of different cultures and customs. Effective communication is critical to a successful outsourcing strategy. If the two companies cannot agree on one point of view or have different understandings of the same word, the relationship is less likely to succeed.
Ultimately, it's important to remember that business is conducted between people. Although the technical expertise and track record of a company stands for itself, the best outsourcing relationships are built on trust, dedication, communication, understanding, and respect.
Pradep Nair is vice president and head of global life sciences and healthcare practice at HCL Technologies Ltd. (Florham Park, NJ). He can be contacted at [email protected].
Reference
1. Frost & Sullivan, “U.S. Medical Devices Market Outlook” (San Antonio, TX: Frost & Sullivan, 2005).
Copyright ©2007 Medical Device & Diagnostic Industry
About the Author(s)
You May Also Like


.png?width=300&auto=webp&quality=80&disable=upscale)
