MX: A Head Start for Medical Device Start-ups
IP protection, and particularly patent protection, is critical to attracting funds for new ventures in the medical device industry. Accordingly, medical device startups developing a filing strategy need to determine how many patents and patent applications their companies require in order to finance each stage of development.
|
Keith D. Lindenbaum |
How should a company determine the number of patents that are necessary before pursuing startup financing, first-stage institutional financing, later-stage institutional funds, or an IPO? The answer to this question depends on a number of factors. They include the specific technology, the specific product, the prior art in the field, and patent trends in the field. However, during the last 10 years we have developed some general guidelines for an effective patent strategy based on our experiences representing medical device startups, established companies considering licensing deals and partnerships with startups, and research into patent portfolios of device companies at the IPO stage. It’s a metric-based approach that could be called the “1-3-15” rule.
One Application for Outside Financing
A new medical device startup typically must own or control at least one U.S. patent application that is pending with the United States Patent and Trademark Office before seeking startup financing from outside investors, (i.e., investors other than the friends and family of the company founder). A pending patent application for the company’s core technology is the first step toward protecting the technology that company will be built on. It stakes out the area of potential patent protection, and it also provides an indication to potential investors of the sophistication of the new company. In addition, the pending patent application allows the new company to disclose aspects of the technology to potential investors without fear of barring patent protection through public disclosure of the technology. The pending patent application also helps to prevent or discourage the potential investor from pursuing the technology on its own without investing in the new company.
|
James D. Borchardt |
Three Marks the Sweet Spot
For the medical device startup seeking intermediate stages of funding, three patent properties mark the sweet spot: one granted U.S. patent, one pending U.S. continuation application, and one pending international (PCT) or European application. Intermediate stages of funding for the device startup can come from various sources. These include VC financing, a partnership with or investment from an established medical technology company, and revenue generated by licensing the technology to an established company.
The granted U.S. patent provides a zone of exclusion for the company’s core technology, and it also indicates that the foreign patent applications also will be granted. Large, established medical technology companies typically have the know-how and resources to independently develop any particular technology. Therefore, the zone of exclusion provided by the granted patent gives the startup leverage to entice an established company into a partnership when the established firm otherwise may not need an association with the startup to pursue the technology.
|
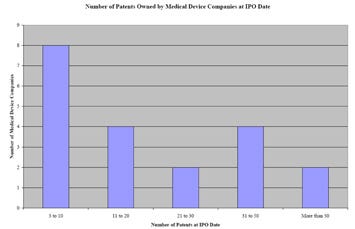
FIgure 1. Snapshot of the number of patents owned by medical device company at IPO. |
The pending continuation application provides flexibility to seek additional patent protection. This flexibility can be critical, because it allows the startup to pursue broader and different patent protection in reaction to changing market circumstances, the development of competing technology by competitors, or changes in the technology as it is integrated into the established company’s existing products. Further, the pending continuation may allow the new company to pursue a patent to cover products that have been designed by a competitor to avoid infringement of the granted patent. For this reason, the pending continuation application accomplishes two important goals. One, it tends to discourage “design around” attempts by potential partners, and, two, it tends to encourage the established company to consider partnering with the startup if the established company wants to pursue the startup’s technology.
Of equal importance, the pending PCT or European application can increase the value of the startup by providing the potential for patent protection in markets outside the United States.
‘Freedom to Operate’—A Plus
In addition to building an appropriate patent portfolio, the medical device startup should conduct a “freedom-to-operate” analysis related to the company’s planned commercial product in order to increase the chance of successfully attracting investment during the intermediate stages. The freedom-to-operate analysis seeks to identify IP (typically patents) owned by third parties that may block the startup’s ability to commercialize the planned product. The analysis provides the startup (as well as potential investors and partners) a level of confidence that the planned product does not infringe the IP of third parties. In addition, early identification of “problem patents” may allow the startup to proactively address any such patent by, for example, designing around the patent, obtaining a patent opinion that the commercial product does not infringe the patent, or seeking a license from the patentee.
The chief aim of the patent portfolio owned by the startup is to prevent or deter introduction of competing products. As such, a strong patent portfolio may provide the startup with an edge in the marketplace by forcing competitors to use less attractive technology, For this reason, the startup’s patent portfolio is appropriately one important factor in its ability to raise capital. Eventually, however, the startup or its partner will need to sell a product in order to generate revenue. The freedom-to-operate analysis helps to ensure that when the time comes for commercialization third-party IP does not provide an unexpected challenge to the sale of the product.
The IPO and 15 Patents
Preliminary research shows that the average medical device company owns a portfolio of about 15 U.S. utility patents at the date of IPO. An examination (with the outliers excluded) of the U.S. patent portfolios of 20 medical device companies that successfully went through an initial public offering in the past 10 years indicates that the median-sized patent portfolio of a medical device company at IPO included 15 granted U.S. utility patents. As depicted in Figure 1, our research also shows that 8 of the 20—or about 40%--of the companies had 3 to 10 U.S. utility patents at IPO and that 20% of companies held 11 to 20 U.S. utility patents at IPO (see Figure 1).
Many medical device products include multiple components and may be one part of a larger system. Thus, the multiple patents in the IPO patent portfolio may be needed to adequately protect the multiple aspects/components/functionalities of the new medical device product. In addition, adequate patent protection for the startup’s core product appears to be an important factor in successfully taking the startup through IPO.
Given the increasingly competitive financing environment, the 1-3-15 IP metrics model provides medical device startups with a tool to gauge their own progress in an area that is important to investors. Because obtaining granted patents takes some time, startups that are developing a business plan for both short- and long-term success should develop a patent strategy early with an eye toward building a patent portfolio based on 1-3-15 IP metrics.
Keith D. Lindenbaum is a partner at Foley & Lardner LLP (Milwaukee) with a focus on IP counseling and management. James D. Borchardt is an associate and registered patent attorney with the law firm.
About the Author(s)
You May Also Like