Functional Imaging and Molecular Radiosensitizers
Molecular biology is poised for an increasing role in radiation therapies for cancer.
COVER STORY: RADIATION THERAPY
|
(click to expand) |
The use of radiation for treating cancer emerged soon after the discovery of x-rays and radioactivity in the late 19th century. In recent years, major advances in tumor imaging and computing power have enabled increasingly accurate delivery of radiation to a targeted volume in the patient. Today, radiotherapy is widely used to treat several cancers and its efficacy compares favorably with other approaches, with demonstrated cost benefits. However, the current arc of development for radiotherapy fueled by improvements in physics and computing power is reaching its technological limits. The next technological step will likely be driven by an adjacent discipline: molecular biology.
Molecular biology provides insights into the specific pathways that allow cancerous tissue to establish a presence, proliferate, fight immune system attacks, and repair damage. Future improvements in radiotherapy efficacy will arise by disrupting the biological repair mechanisms that some resistant cancer cells deploy in response to irradiation. This emerging field of molecular radiosensitizers, and its consolidation with functional imaging, enables insight into tumor biochemical activity beyond standard anatomical imaging and promises to dramatically change radiotherapy.
The commercial potential of molecular radiosensitizers and functional imaging is well demonstrated by the emergence of multi-million-dollar alliances and several new companies. As results from current clinical trials are published and disseminated, innovation and clinical acceptance for this new field should increase dramatically.
Radiotherapy for Cancer
Residents of industrialized countries have a 30–50% chance of developing cancer over their lifetimes, and more than half of them will receive radiotherapy as part of their treatment.1,2 In the United States alone, radiotherapy was used to treat a million patients in 2004, and its increasing adoption has been accompanied by a significant growth in the number of treatment facilities.2 "General Principles of Software Validation; Final Guidance for Industry and FDA Staff," (Rockville MD: FDA) January 11, 2002. 1,3 In many countries with public healthcare insurance, the infrastructure for radiotherapy has been unable to satisfy demand. For example, in the UK, more than half the patients eligible for radiotherapy wait for longer than the recommended maximum period of four weeks due to a shortage of facilities.4
|
Table I. (click to expand) Selected five-year disease-free survival rates of cancer patients in the United States by treatment modality. Compiled from various sources including Merck Manual Online and the National Cancer Institute. |
Currently radiotherapy is one of three major tools for treating cancers, along with surgery and chemotherapy. As a stand alone treatment, radiotherapy has demonstrated efficacy similar to other approaches for selected cancers. Its use in combination with other approaches is standard treatment for many advanced cancers (see Tables I and II).
|
Table II. (click to expand) Overview of radiotherapy modalities. |
Current radiotherapy modalities use external beams, internal implants, or circulating tagged radionuclides to deliver ionizing radiation with the aim of preferentially killing tumor cells. More than 90% of radiotherapy patients are treated with external-beam radiotherapy (EBRT), the use of which has been spurred over the last two decades by techniques to deliver radiation doses to tumors with a high accuracy. Within EBRT, 3-D conformal radiotherapy (3DCRT) is the standard of care in most industrialized countries. This therapy uses 3-D tomographic images to design megavoltage x-ray beams that conform to the tumor volume, minimizing the dose to healthy organs and tissue. A recent innovation in EBRT is intensitymodulated radiotherapy (IMRT), introduced in 1999, which is supplanting 3DCRT primarily in the United States. IMRT deposits a large, specified radiation dose into a targeted volume by changing beam shapes and cross-sectional profiles at each angle of incidence. Medicare reimbursement championed IMRT by paying nearly $30,000 for a course of treatment, almost three times the payment for 3DCRT. Strong growth for IMRT is expected in Europe due to its recent positive clinical evaluations by national health systems.5
Image-guided radiotherapy (IGRT), the successor to IMRT, uses imaging approaches incorporated into radiation delivery in real-time to dynamically track changes in tumor location and shape during treatment. The incremental clinical benefit of IGRT over IMRT is yet to be established and Medicare is reimbursing only 20% more for IGRT protocols. Similarly, arc/helical therapy, delivered by a beamlet of radiation rotating around the patient, has yet to demonstrate improved clinical outcomes that would spur displacement of existing systems.
Stereotactic radiosurgery uses an external 3-D reference frame that is physically attached to the patient to precisely deliver radiotherapy with multiple beams. It primarily treats intracranial lesions, but is being extended to other areas of the body such as the lungs, liver, and pancreas.
Brachytherapy and therapeutic radiopharmaceuticals both provide internal sources of radiation. Their future use (and that of cobalt 60 external beam sources) could be affected by rising security concerns over the transport of radioactive material. Brachytherapy uses sealed sources as localized implants. While patients with low-dose-rate implants are not constrained, radiotherapy with high-dose-rate seeds is done using special catheters for limited intervals as an outpatient procedure. The common patient preference for a painless, noninvasive procedure restrains the use of brachytherapy, and favors external-beam radiotherapy.
The radiopharmaceutical iodine-131 has been used for thyroid treatment since 1946. For decades, radioimmunotherapy, involving tumor-targeting antibodies labeled with radioactive isotopes, has generated significant expectations. However, in contrast to the success of targeted immunotherapies, radioimmunotherapy products for lymphomas have not been commercially successful because their use requires an infrastructure involving nuclear pharmacies that is typically available only in large teaching hospitals.6
The Radiotherapy Market
The total market for radiotherapy is composed of two major elements: professional services and products. Services include charges for the professional medical staff such as radiation oncologists, medical physicists, dosimetrists, nurses, and facility administrators. Given the tremendous disparity in healthcare systems across the globe, professional service charges vary greatly and are difficult to compare. Charges for products, however, are more uniform and can be tracked easily. The total value for radiotherapy products in 2007 was $2.6 billion at manufacturer prices. The equipment is composed of linear accelerators (linacs) and specialized imaging equipment (simulators), specialized treatment planning software, internal radiation (brachytherapy) sources, and radiopharmaceuticals. Historical (2004–2007) and projected (2008–2011) growth rates for the radiotherapy market are 6.8% and 7.0%, respectively. Published market forecasts ascribe growth in the cancer market to immuno- and biotherapies, taking the view that radiotherapy products will continue to be expensive hardware such as linacs.
The Multidisciplinary Approach to Curing Cancer
|
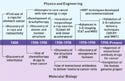
Figure 1. (click to expand) Historical development of physics and biology approaches for cancer. |
The development of radiotherapy has been traditionally led by physics and engineering. As shown in the time line in Figure 1, the first attempts at curing cancer using ionizing radiation started almost immediately after the discovery of x-rays and radioactivity in the 1890s. The principles of radiation's effect on biological tissues began to take shape at the turn of the 20th century, with empirical observations on the effect of x-rays on cells that correlated their proliferative activity and radiosensitivity. A major technological leap in the 1950s was the switch from low-energy x-rays that severely damaged the skin to cobalt 60 sources and linacs providing megavoltage x-rays with deep penetrating power. Radiotherapy received a tremendous boost following the advent of computed tomography (CT) imaging in the 1970s. CT had tremendous computing power to generate 3-D images that could be used for effective treatment planning.
In contrast, molecular biology is a more recent scientific discipline that blossomed after the structure of DNA was discovered. It now plays a fundamental role in elucidating both the basis of radiotherapy and molecular pathways underlying the various cancers.
Future Trends in Radiotherapy
The Physics Approach Gets More Expensive. The next-generation advance in radiotherapy involves the use of heavy particles for a greater precision in dose delivery at a targeted depth inside the patient. Biological damage from megavoltage photons is spread over larger distances compared with damage caused by heavy particles such as protons or carbon ions. A more precise radiation dose delivery not only promises greater efficacy in eradicating the targeted tumor, but it also reduces side effects including the risk of secondary tumor formation in overirradiated normal tissue. Such increased precision comes at a price, however. Constructing a facility for delivering radiotherapy with heavy particles is estimated to cost more than $100 million.7 Despite some enthusiasm and deep pockets among academic medical centers in the United States, it is unlikely that more than a handful of these types of facilities will be built around the world.
Imaging Highlights the Greater Role for Biology. While the delivery of radiation dose has become increasingly precise, accuracy in specifying the clinically significant tumor volume continues to drive developments in imaging. CT has been widely adopted in radiotherapy because it provides an accurate 3-D map of tissue density information that is an accurate basis for calculation of radiation dose. It also enables simulation of the radiotherapy process on the patient. Magnetic resonance (MR) provides more soft-tissue contrast, and in many instances does a better job of tracking spatial changes in the tumor during radiotherapy. Integrating different imaging techniques has required significant investment. It calls for upgrades to software that can seamlessly combine images from multiple modalities.
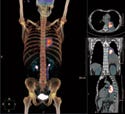
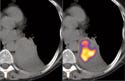
Positron emission tomography (PET) has extended the role of imaging to include observation of tumor biochemical pathways in real-time.8 PET requires injecting imaging agents that contain a positron emitter. By localizing the origins of the two identical gamma rays traveling in opposite directions that result from each emitted positron's annihilation with an electron, users can map the PET radiotracer distribution.
|
The most common PET tracer is the glucose analog 18F-fluorodeoxyglucose (FDG). While CT and MR provide anatomic images, FDG-PET provides a functional map that can be used for tumor staging and therapy monitoring. In particular, lung cancer, a disease for which the overall five-year survival rate is below 10%, has seen increased application of PET scanning for early tumor detection. Tumors consume abnormally high amounts of glucose (and FDG), which makes them visible with FDG-PET.
Combined PET/CT scanners provide an accurate overlay of anatomic and functional images, with an imaging time of 5–10 minutes, which is significantly below the 45 minutes required by PET-only devices. PET/CT scanners now comprise 90% of PET equipment sales.9
|
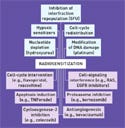
Figure 2. (click to expand) Classical (top) and current (bottom) strategies for radiosentization. |
A critical enabling technology for use of PET in identifying cancer is the development of injection targets combined with tracers. Monoclonal antibodies (mAbs) tagged with positron emitters that target individual biomarkers have been a promising innovation. Thus far, FDA has approved 21 mAbs for therapy and five for imaging, largely in oncology.10
Different Radiation Doses for Different Tumors. The typical cellular response to radiation includes pathways in DNA repair, control of cell-division cycle checkpoints, programmed cell death (apoptosis), and signal transduction.11 In addition to these typical responses, cancer cells have unique adaptations such as the ability to promote growth of blood vessels (angiogenesis), and function in oxygen-starved environments (hypoxia).12,13 Each pathway provides targets for optimizing the radiation response by either blocking the pathway the cancer cell is using to repair the radiation damage or sensitizing the cancer cell to be more vulnerable to the radiation it is about to receive.
The goal of developing radiosensitizers is to optimize the killing dose of radiation for the specific tumor being targeted and to inhibit the specific pathways the tumor uses to repair itself. This may vary by the life stage of the tumor as well. The need for intelligence on the specific pathways being exploited by a tumor underscores the forementioned close relationship of radiotherapy with functional imaging.14
An overview of the current development strategies for molecular radiosensitizers is shown in Figure 2.15 Many of these approaches are currently being tested in clinical trials. Key examples of molecular radiosensitizers in development include the following:
Hypoxia confers resistance to radiation on cancer cells. A Phase III clinical trial for advanced head and neck cancer combines accelerated radiotherapy with carbogen (a hyperoxic gas) and nicotinamide (a vasodilator), which could enhance oxygen delivery to a tumor.16
GenVec (Gaithersburg, MD) is conducting a pivotal Phase II/III trial with TNFerade in patients with locally advanced pancreatic cancer. TNFerade is a modified virus that contains the gene for tumor necrosis factor-alpha, an immune system protein with well-documented anticancer effects, which is injected into tumors for use in combination with radiation, chemotherapy, or both.
Epidermal growth factor receptor (EGFR) is often over-expressed by tumor cells, and blocking it sensitizes the cells to radiation. Targeting EGFR using Erbitux (cetuximab) in patients with advanced head and neck squamous cell carcinoma significantly improved overall patient survival in a randomized pivotal trial.16
Avastin (bevacizumab) binds vascular endothelial growth factor (VEGF) molecules and inhibits angiogenesis, the mechanism by which tumors recruit new blood vessels. The effects of combining Avastin and other angiogenesis inhibitors with radiotherapy are being investigated. Inhibiting a tumor's blood supply can also make it hypoxic and less radiosensitive.17
|
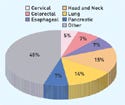
Figure 3. (click to expand) Ongoing clinical trials investigating radiosensitization grouped by tumor site. |
There are more than 200 clinical trials under way that investigate the use of radiosensitization (see Figure 3). Not surprisingly, the greatest number of trials is for cancers that have shown significant resistance to surgical, chemotherapy, and radiotherapy treatments. Nearly all are treated with existing chemotherapy drugs that may demonstrate radiosensitization properties. The development of new radiosensitizing agents is generally in the preclinical stage.
Market Potential of Radiosensitizers
|
(click to expand) |
The market for radiosensitizers is potentially larger than the current total radiotherapy equipment market. The market estimate is built by analyzing those cancers for which radiotherapy is prescribed today and have less than 50% five-year survival rates. There are more than 500,000 patients in the United States alone that could qualify for radiosensitizers. At a price of $7200 per radiosensitizer (the weighted average cost of chemotherapy treatments), the total U.S. market potential is more than $3.7 billion. The potential for any one radiosensitizer would be a fraction of this opportunity. However, by comparison, the global market for all radiotherapy products in 2007 was $2.6 billion. This is clearly a high-growth potential market that has opportunities for multiple types of players including manufacturers of radiotherapy and diagnostic imaging equipment, small biotech firms, and big pharmaceutical companies.
Conclusion
Radiotherapy is in transition from being an empirical discipline driven primarily by physics to a clinical science founded in an understanding of molecular pathways. There is a strong interest in the oncology community to extend the successes of radiotherapy across various cancers by validating novel markers with functional imaging and exploiting molecular mechanisms underlying radiosensitivity. The current level of activity, with clinicians validating existing chemo- and targeted therapeutic agents for use with radiotherapy, suggests that radiotherapy will become a promising area for drug development within a few years. The market opportunity is expected to be very significant, given the essential role of radiotherapy in treating cancer.
Usman Qazi is a principal at Scientia Advisors (Cambridge, MA), a consulting firm with a concentration in life sciences and healthcare. He can be reached at [email protected]. Amit Agarwal is a partner at the firm, and he can be contacted at [email protected].
References
1. “Targeting Cancer Care, Annual Report 2006–2007,” [online] (Fairfax, VA: American Society for Therapeutic Radiology and Oncology [cited 14 July 2008]) available from Internet: www.astro.org/AboutUs/AnnualReports.
2. NG Burnet et al. “Improving Cancer Outcomes Through Radiotherapy,” British Medical Journal 320 (January 2000): 198–199.
3. Leslie K Ballas et al., “Radiation Therapy Facilities in the United States,” International Journal of Radiation Oncology, Biology, Physics 66, no. 4 (2006): 1204–1211.
4. Ruth H Jack et al., “Radiotherapy Waiting Times for Women with Breast Cancer: A Population-Based Cohort Study,” BMC Cancer 7 (May 2007): 71.
5. Haute Autorité de Santé (France), “Radiothérapie Conformationelle avec Modulation d'Intensité,” 2006.
6. Ken Garber, “Users Fear That Lymphoma Drugs Will Disappear,” Journal of the National Cancer Institute 99 (April 2007): 498–501.
7. B Jones, “The Case for Particle Therapy,” British Journal of Radiology 79 (2006): 24.
8. Tom Blodgett et al., “PET/CT: Form and Function,” Radiology 242 (2007): 360–385.
9. Van Dongen et al., “Immuno-PET: A Navigator in Monoclonal Antibody Development and Application,” Oncologist 12 (2007): 1379–1389.
10. David S Boss et al., “Application of PET/CT in the Development of Novel Anticancer Drugs,” Oncologist 13 (2008): 25–38.
11. Philip J Tofilon et al., “Molecular Targets for Radiation Therapy: Bringing Preclinical Data into Clinical Trials,” Clinical Cancer Research 9 (2003): 3518–3520.
12. Henning Willers and Kathryn D Held, “Introduction to Clinical Radiation Biology,” Hematology/Oncology Clinics of North America 20 (2006): 1–24.
13. Harrington et al., “Molecular Biology for the Radiation Oncologist: The 5 Rs of Radiobiology Meet the Hallmarks of Cancer,” Clinical Oncology (Royal College of Radiologists) 19 (2007): 561.
14. CN Coleman, “Linking Radiation Biology and Imaging Through Molecular Biology,” Radiology 228 (2003): 29.
15. Eric Deutsch et al., “New Concepts for Phase I Trials: Evaluating New Drugs Combined with Radiation Therapy,” Nature Clinical Practice Oncology 2 (2005): 456–465.
16. Juliette Thariat et al., “Integrating Radiotherapy with Epidermal Growth Factor Receptor Antagonists and Other Molecular Therapeutics for the Treatment of Head and Neck Cancer,” International Journal of Radiation Oncology, Biology, Physics 69, no. 4 (2007): 974–984.
17. Suresh Senan and Egbert Smit, “Design of Clinical Trials of Radiation Combined with Angiogenic Therapy,” Oncologist 12 (2007): 465–477.
Copyright ©2008 Medical Device & Diagnostic Industry
About the Author(s)
You May Also Like