WASHINGTON WRAP-UP
August 1, 2006
|
According to acting FDA commissioner Andrew von Eschenbach, “FDA is committed to being strategically placed between discovery and development. We're not here just to regulate, but also to facilitate.” Von Eschenbach addressed the Medical Device Manufacturers Association (MDMA) annual meeting on June 14. “Basic scientific understanding at the molecular, genetic, and cellular levels has opened up new opportunities to modulate the disease process,” he continued. “And a better understanding of the basic mechanisms of the disease process can enable us to alter the process and can lead to more-effective interventions.” He said the future of FDA lies in its ability to provide an infrastructure or bridge leading from discovery to development of new drugs and devices.
As a former practicing urologist, von Eschenbach described himself as “by definition, a gadgeteer.” He noted that he has personally witnessed the importance of medical devices as key elements in promoting the health and welfare of patients. He predicted that over the next decade device innovation would result in less-invasive medical interventions and better and more-rapid patient recoveries. He cautioned, however, that “giant leaps” as well as “incremental improvements” in device technologies could only occur conditionally. Basic researchers, product developers, regulators, and medical practitioners must maintain a continual dialogue. “The entire process from discovery to product development must become more integrative, more collaborative,” he said.
Von Eschenbach also discussed the advantages to be gained by such an approach. He described the positive experience at Vanderbilt University, where basic research to identify biomarkers associated with various cancers was transferred from the medical school to the school of engineering. “With the transfer, the pace of biomarker discovery accelerated, [thanks to] the engineers' expertise in mass spectrometry. In turn, the engineers gained a better understanding of requirements of the medical community. There quickly developed a highly productive symbiotic relationship.” He further noted that the computing capability of Oak Ridge National Laboratory has recently been made available to Vanderbilt researchers. He predicted that those capabilities would amplify the opportunities for advances in biomarker development and technologies. “FDA very much wants to be a part of this kind of important collaboration and dialogue,” he said.
CDRH director Daniel Schultz followed von Eschenbach's presentation with an update on emerging trends in the field of medical devices as indicated by regulatory submissions to the agency. He also described the center's current activities. According to Schultz, a wide variety of innovative products have been received for review. Products include computer-related technologies, wireless systems, and minimally invasive technologies. Other products submitted related to molecular medicine, including devices used in genomics, proteomics, gene therapy, bioinformatics, and personalized medicine. Others comprise robots for in vitro sample handling, superhigh-spatial-precision surgery, and prosthetics. Products for organ replacements and assists and decentralized healthcare (technologies related to home care and telemedicine) were also received.
CDRH is also pursuing research projects to assist industry in conducting clinical trials. Among the more interesting projects is the creation of a so-called virtual family. It's a simulation-based engineering and medical imaging technology for cardiovascular and peripheral vascular stenting device development. Under this concept, members of the virtual family have simulated vascular systems that vary by sex, age, and other key characteristics. The members could be enrolled, so to speak, in virtual clinical trials to help determine the value of new or innovative stent designs. In theory, this technique could reduce the need for human subjects in early clinical trials.
Schultz spoke in positive terms about the beneficial effect of user fees on CDRH and on its ability to respond more quickly to device review applications. He said that budget relief provided by user fees has allowed CDRH to hire a number of “young, talented people.” Many of those hired have expertise in the areas of engineering, medical science, and statistics.
Schultz also pointed to the development of electronic tracking systems for review documents. Also beneficial is e-Consult, an in-house electronic system that enables FDA reviewers to coordinate reviews from various office locations, thereby saving review time. Finally, the deployment of the Turbo 510(k) program by which applications can be received and processed electronically is a big step. On the latter point, Schultz noted that more than 50 e-submissions have been received by CDRH and reviews of 30 of these have been completed. And the pace of e-submissions is accelerating; more than two-thirds of the total received have come in during this calendar year.
Schultz offered a spirited defense of FDA's use of outside advisory panels in response to critical comments from the audience. Members of the audience felt that “the work of years can be undone in one afternoon” by panel members who possess “inadequate expertise” or the “wrong background” to pass judgment on a particular medical device or technology. Schultz asserted that “a strong outside advisory group is vital to the way we do business. A public process to air scientific issues—as afforded by public advisory panel sessions—is critical to FDA's overall mission of getting innovative technologies to the marketplace.” He agreed, however, that it is incumbent on FDA to seek out and employ the right experts to serve on the panels. FDA must also provide all necessary information on products to be considered in time for their careful review, prior to advisory panel meetings.
Schultz also echoed sentiments expressed a month earlier by his counterpart in FDA's Center for Drug Evaluation and Research. He noted a special concern over current discussions related to alleged conflict-of-interest issues pertaining to advisory committee members. The implications are that too strict an interpretation of the term conflict of interest could deny the agency the expert advice it needs. “Depending on how this discussion goes, it could present major problems for the agency,” he said.
FDA Considers Bar Codes for Devices
An FDA-commissioned report suggests ways FDA and the device industry can move toward implementing standardized bar coding–like unique device identification (UDI) technology. Such coding may help cut down errors, facilitate recalls, improve inventory control and reimbursement, and reduce product counterfeiting. In 2004, FDA rolled out its regulation on bar coding on pharmaceutical labeling. At that time, the agency decided to defer any related system for devices until certain issues were properly studied. One of the primary roadblocks for devices, according to FDA at the time, was that the industry lacked a standard and unique identifying system comparable to the National Drug Code system for pharmaceuticals.
FDA now is reconsidering whether some form of UDI is warranted for medical devices, according to the report. Prepared by Eastern Research Group, the report outlines five steps for consideration in implementing UDI for medical devices (see below).
UDI for Medical Devices
A report prepared by Eastern Research Group lists five steps to be considered when implementing UDI for medical devices:
• Select a unique identifier.
• Identify the data needed for patient safety.
• Determine how hospitals will use UDI.
• Standardize and synchronize product data (based on the unique identifier).
• Maintain a central repository of the standardized data.
FDA Ombudsman Resolves More Complaints
|
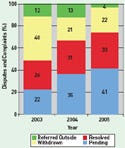
Figure 1. (click to enlarge) The CDRH ombudsman resolved more disputes in 2005 than in 2003 or 2004. |
CDRH ombudsman Les Weinstein resolved slightly more complaints and disputes in 2005 than in 2003 or 2004, and the number of new complaints and disputes received dropped (see Figure 1). The information was presented in Weinstein's annual report for calendar year 2005. The data indicate that 41% of complaints and disputes received in 2005, plus those carried over from previous years, were pending at year's end. Thirty-three percent were resolved, 22% withdrawn or not followed up by the complainant, and 4% referred outside CDRH. In 2004, the breakdown was 35% pending, 31% resolved, 21% withdrawn, and 13% referred outside. And in 2003, it was 22% pending, 26% resolved, 40% withdrawn, and 12% referred.
There were a total of 114 complaints, disputes, and other issues received by the ombudsman in 2005, compared with 125 in 2004 and 120 in 2003. Industry was the source of most of the submissions (75%), with 17% coming from consumers and 4% from healthcare providers. The remaining complaints came from various other categories.
The most common reason for complaints and disputes remains miscommunication or lack of communication, as it has been each year. The second- most-common reason was disputed data or testing requirements to support a submission and the least burdensome approach, the same as in 2004. Lack of timeliness in approvals, setting meetings, and returning phone calls moved up from number four in 2004 to number three in 2005. The biggest jump was safety concerns and issues, which moved from eighth place in 2004 to fourth place in 2005.
FDA Revamps Annual Report Rules
CDRH's Office of Device Evaluation (ODE) is working on a Level 1 guidance that would bring consistency to annual reports filed by premarket approval (PMA) sponsors. ODE PMA program director Thinh Nguyen reported this news to the Orange County Regulatory Affairs (OCRA) Discussion Group's annual meeting with FDA on May 24. The proposed guidance, which will soon be published for comment, will seek to establish separate sections within a standardized format for manufacturing, design, and labeling changes during a reporting period. The format will be designed to facilitate linkages between reported labeling changes and medical device reports (MDRs) received.
Nguyen told OCRA his office wants to see the sponsor's rationale for why a reported change did not affect the safety and effectiveness of the device. He also wants to see sufficient evidence, including risk analysis, and a summary of the sponsor's validation or verification activities.
Rationales, he said, should explain the result of a problem. For example, did the problem generate MDRs (give MDR numbers), was it an adverse event or defect, or was it associated with a recall or corrective action? Sponsors should also explain whether a change was in response to a customer complaint, request, etc., and whether it was related to any public disclosure on the manufacturer's part (“Dear Doctor” or “Dear Patient” letters). Also, sponsors should disclose whether the change was related to any FDA safety alert or warning letter.
If a change is associated with a failure or a problem, Nguyen said, the sponsor should describe its investigation of the cause or source of the problem. It should also explain its decision to change the device and describe any actions taken to correct the problem or mitigate harm.
ODE will be proposing a separate section in the annual report for information on clinical postapproval studies. That section will include progress so far, or any results, and the date on which the postapproval study report was submitted to FDA.
Nguyen acknowledged to OCRA that modular PMA review times have become unpredictable. “The program is not working effectively,” he said, “It is supposed to be designed so that you submit a module, close it out, submit another module, close it out, then come in with the PMA. What we have is three or four different modules open, and then the company deciding, ‘I'm not going to answer those [questions] raised by FDA in the module. I'm just going to go ahead and submit the PMA. So now we have three different open applications that we have to look at. It makes it extremely difficult for us.”
Nguyen also acknowledged that module review times continue to run past the 90-day goal, but said additional resources will be needed. A letter to Congress accompanying the Medical Device User Fee and Modernization Act of 2002 said that industry and FDA were to work together to establish performance goals for modular reviews, he said. Several years ago, FDA suggested some goals, but industry proposed alternatives and “since then, we haven't come to an agreement.”
On approval-pending GMP letters, Nguyen said the PMA review clock stops on issuance. “From that point forward, it's between the Office of Compliance, the field, and the company to work out when the manufacturing facility is going to be acceptable.” This raises a problem for industry, he said, because often firms say FDA inspectors do not come even though the site is ready and waiting. And then companies feel the review clock should be turned back on. However, sometimes the company is not ready for FDA.
Nguyen said the only solution is to change the approval-pending GMP letter. It should be clear that the review clock's resumption depends on who is at fault for the inspection's delay.
Study Finds 20% of Defibrillators Are Recalled
By the Numbers: 1996–2005 FDA advisories issued: 52 *during attempted resuscitation of cardiac arrest victims |
Over the past 10 years, more than 20% of automated external defibrillators (AEDs) were recalled, attendees of the Heart Rhythm Society's 27th Annual Scientific Session in May were told. The data came from a study led by FDA Circulatory System Medical Device Advisory Panel chair and Beth Israel Deaconess Medical Center physician William H. Maisel. The study reviewed FDA enforcement reports and safety alerts from 1996 to 2005. Electrical and software problems were the most common reasons for recalls and advisories being issued. During the study period, the authors found that FDA issued 52 advisories involving either AEDs or critical AED accessories, affecting a total of 385,922 devices. The authors note that the annual number of devices distributed between 1996 and 2005 increased almost tenfold, from fewer than 20,000 to nearly 200,000. In a separate analysis, the authors found that during the same 10-year period, 370 confirmed AED malfunctions occurred during attempted resuscitation of cardiac arrest victims.
“While AED malfunctions do occasionally occur, the number is small compared with the number of lives saved by these important devices,” notes Maisel in a release from the medical center. However, he continues, the number of AEDs in distribution continues to increase rapidly. In response, efforts should be directed at developing a system to find and fix potentially defective devices quickly.
The Heart Rhythm Society issued a separate report in April on postmarketing safety studies. It called for advisory committees to begin reviewing such safety reports and making recommendations on recalls involving cardiac rhythm management devices. Meanwhile, CDRH director Dan Schultz said at an FDA centennial celebration in May that the agency plans to expand its advisory committee reviews. Over the next several months, they will begin to include postmarketing data and device malfunctions.
Tri-State Hospital Supply Warned by FDA
FDA's Atlanta and Detroit district offices have each sent a warning letter to Tri-State Hospital Supply Corp. over GMP problems. The offices found these problems during inspections at two of the firm's manufacturing and reprocessing facilities.
A May 3 warning letter from the Atlanta district covered a January 18–26 inspection at Tri-State's Salisbury, NC, facility. There, the company reprocesses various types of surgical stainless-steel instruments such as scissors, forceps, hemostats, and needle holders.
The letter said the company failed to validate a process whose results cannot be fully verified by subsequent inspection. It also failed to test and approve its validation according to established procedures. The warning letter acknowledged a response from the company's director of quality assurance and regulatory affairs, but said that the response was inadequate. According to the office, the firm did not give specific information on how the company would validate the process for the affected instruments.
A June 2 warning letter from the Detroit district dealt with a January 18–February 3 inspection at the firm's Howell, MI, facility. That facility manufactures circumcision clamps and reprocesses circumcision clamps and floor-grade surgical instruments such as hemostats, forceps, scissors, and needle holders. The letter said the company's response to the cited items (see below) was inadequate because it did not provide specific information on steps taken.
The Michigan Warning Letter
The inspection at Tri-State's Howell, MI, facility found the following GMP deviations:
• Failure to adequately validate a process whose results cannot be fully verified by subsequent inspection and test.
• Failure to review and evaluate the process and perform revalidation where appropriate when changes or process deviations occur. In addition, the company failed to document such activities.
• Failure to establish and maintain process control procedures that describe process controls to ensure conformance to specifications.
• Failure to monitor and control process parameters during production.
• Failure to establish and maintain adequate procedures for receiving, reviewing, and evaluating complaints by a formally designated unit.
• Failure to establish and maintain procedures for management review.
• Failure to establish and maintain procedures for implementing corrective and preventive actions, including requirements for investigating the cause of nonconformities relating to product, processes, and the quality system.
Copyright ©2006 Medical Device & Diagnostic Industry
About the Author(s)
You May Also Like