Custom IV assemblies
June 1, 1997
Spotlight on IV Components Custom IV assemblies
Custom IV assemblies

A company designs and manufactures custom bag assemblies, IV assemblies, and precision injection-molded components to exact specifications. It uses the latest materials and technologies and emphasizes a team concept of manufacturing from initial design to the finished product. Also available is a sterilizable medical film with good barrier properties and no leaching plasticizers. Advanced Scientifics, 163 Research Ln., Millersburg, PA 17061. (717/692-2104)
 Disposable needleless valves
Disposable needleless valves
Needleless valves limit the risk of infection to health-care providers by replacing the hypodermic needle ports. A company's needleless line consists of a variety of low-priming-volume disposable parts including slip luer valves, Y ports, T ports, 1/4-in. tubing valves, and ANSI standard valves. These components are available in a variety of gamma-, EtO-, or autoclave-sterilizable materials. Halkey-Roberts Corp., 11600 9th St. N., St. Petersburg, FL 33716. (800/303-4384)
 Luer-activated valves
Luer-activated valves
Designed for needleless access systems where fluid delivery is vital, luer-activated valves feature an enhanced design that is compatible with most drugs, including lipids, and provides for the safe inspiration, aspiration, and complete flushing of blood and other viscous fluids. The LAV+ valves feature a preloaded nonlatex diaphragm that checks flow until mechanically opened with a luer. This provides dependable fluid-flow control to protect both patient and clinician. The valves are composed of an elastomeric diaphragm and a sonically bonded thermoplastic body that is pressure tested to 45 psig. Materials meet USP Class VI requirements and can be gamma or EtO sterilized. NP Medical Inc., 101 Union St., Clinton, MA 01510. (508/365-2500)
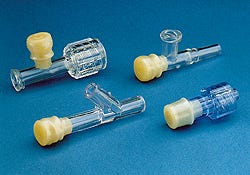 Latex-free injection sites
Latex-free injection sites
With good reseal, a family of latex-free injection-site components offers all the properties of natural rubber without the contaminants, thereby eliminating the possibility of natural rubber latex sensitivity reactions. Components include Y injection sites, T connectors, and sampling ports. Made of 100% synthetic polyisoprene and available in a standard gold color, they are sterilizable by EtO or gamma irradiation. Harmac Medical Products Inc., 2201 Bailey Ave., Buffalo, NY 14211. (716/897-4500)
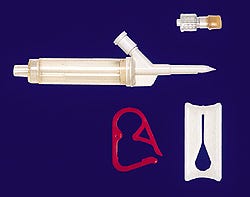 IV components and tubing
IV components and tubing
A company offers a variety of IV components including injection sites, spikes, chambers, clamps, and connectors. A spike-and-chamber assembly with ball check valve protects the line if the setup is tilted. Pinch, slide, and roller clamps are available for flow control applications. The company also carries tubing in more than 100 stock sizes as well as various other components. Samples and a catalog are available. Qosina, 150-Q Executive Dr., Edgewood, NY 11717. (516/242-3000)
 Custom IV components
Custom IV components
A manufacturer of disposable medical devices offers a variety of IV components. Its product line includes male and female luer adapters, stopcocks, check valves, single- and dual-flow piercing devices, barbed connectors, clamps, caps, Y fittings, T fittings, and tri-connectors. Custom designs are available. Burron OEM Div., B. Braun Medical Inc., 824 Twelfth Ave., P.O. Box 4027, Bethlehem, PA 18018. (800/359-2439)
 Fluid-system connectors
Fluid-system connectors
Quick-disconnect couplings for use in low-pressure fluid-management systems are available in a wide range of sizes and styles. Connectors from more than 20 product families find applications in devices and systems for vascular compression, body-temperature control, noninvasive blood pressure monitoring, thoracic surgery drainage, laparoscopy irrigation and insufflation, blood assays, stomach pumping, urine collection, autotransfusion, vital-signs monitoring, and cardiopulmonary procedures. Products are manufactured in controlled environments with specialized protocols for material handling, packaging, and assembly. Custom designs are a specialty. The company is ISO 9001 certified. Colder Products Co., 1001 Westgate Dr., St. Paul, MN 55114. (800/492-0091)
 IV components
IV components
Standard sleeve stoppers, flashback bulbs, and metal seals for IV systems are available for a variety of needs and can be provided quickly and in large volumes. The manufacturer frequently provides custom designs that meet the requirements of various infusion dispensing systems. Analytical support is offered for unique formulation requirements including evaluation of coatings and laminates. Latex-free formulations are available. The West Co., 101 Gordon Dr., Lionville, PA 10341. (800/231-3000)
 One-way check valve
One-way check valve
A one-way, normally closed, minimal-cracking-pressure check valve uses a patented sealing mechanism that eliminates backflow without sacrificing forward-flow performance. Drug use is maximized through a unique internal housing design that allows for complete solution recovery. The valve is available with male/male and male/female connections for compatibility with all IV products using ANSI standard connectors. Composed of medical-grade materials, the valve can be gamma or EtO sterilized. Filtertek Inc., P.O. Box 310, Hebron, IL 60034. (815/648-2461)
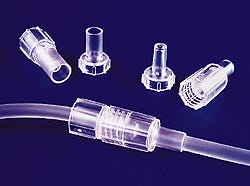 Large-bore shielded connectors
Large-bore shielded connectors
Large-bore tubing connectors are joined together by a luer-type tapered fit to provide secure, leak-free connections. A protective sleeve around the tapered sealing surface eliminates the risk of touch contamination. The connectors protect health-care personnel from exposure to bodily fluids and also protect the patient by guarding the system's sterility during disconnects. Made of clear, medical-grade acrylic, the connectors can be gamma or EtO sterilized. Resenex Corp., 9614-F Cozycroft Ave., Chatsworth, CA 91311. (818/882-7518)
 Rubber components
Rubber components
A supplier of molded rubber components offers a product line that includes stock and custom-designed components. Latex-free and silicone-free compounds are available. Latex-free formulations have been used to produce injection sites and septa that do not produce protein reactions in patients. Other developments include halobutyl stoppers that require minimum amounts of silicone, yet are extremely low in extractables. Tompkins Rubber Co., P.O. Box 160, 550 Township Line Rd., Blue Bell, PA 19422. (610/825-3400)
You May Also Like

