Copolyester Is Clear Choice for Safety Syringe
January 12, 2004
OriginallyPublished MPMN January 2004PROFILE
Copolyester Is Clear Choice for Safety Syringe
The material's tribological properties enhance product's passive activation design
Rita Emmanouilidou
|
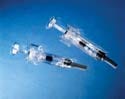
The UltraSafe passive delivery system protects healthcare practitioners from accidental needle sticks. The device's inner body is made from Eastar copolyester DN003, a material with minimal friction that offers the strength and clarity of polycarbonate. |
The advent of needle-safety devices received a boost in 2000 with the enactment of the OSHA Needle Stick Safety and Prevention Act. Safety Syringes Inc. (SSI; Carlsbad, CA; www.safetysyringes.com) was ahead of the curve when it introduced the manually activated Ultrasafe Needle Guard in 1999. It recently introduced a passively activated design. In developing its newest product, the company sought a combination of enhanced safety and aesthetics. The use of Eastar copolyester DN003 from Eastman Chemical Co. (Kingsport, TN; www.eastman.com)has helped the firm to achieve its goal.
The UltraSafe passive delivery system comprises two molded components: the outer guard, which shields the user from accidental needle sticks, and the inner body. The outer guard has a grip that is automatically activated afterinjection. "When the plunger bottoms out, the syringe slides back into the body of the device," says Erik Miller, SSI director of marketing. "The needle is then covered by the guard portionof the device."
During the design phase, the company determined that the outer guard had to have the same look and feel as the inner body. To reduce friction and ensure the timely release of the protective guard, however, each component had to be molded from a different material. Polycarbonate (PC) was selected for the outer guard. The remaining challenge was to find a material for the inner body that had the strength and clarity of polycarbonate while minimizing friction.
Eastar copolyester DN003 fit the bill. The material did not cause undue friction, and it provided the mechanical functionality required to activate the safety device. DN003 resists chemicals and can withstand most sterilization methods. Additionally, it processes similarly to polycarbonate, saving SSI the expense of potential mold modifications and added production costs. "There were no negatives to making the change," says Tom Hall, SSI's regulatory affairs and quality assurance director. "DN003 was virtually a drop-in replacement. The form and function of the part stayed the same."
Eastar copolyesters are suited for a range of medical applications. The materials are durable, withstanding stress and impact. Because of their ductility, they can be used in snap-fit assemblies, thus eliminating solvent bonding. They can be extruded and injection or blow molded, and are available in water-clear or colored formulations. Customers can select the material with only those properties required for their medical product application.
SSI's UltraSafe passive delivery systems can accommodate a broad range of syringesizes and staked or luer lock/slip needle configurations. They are suitable for use with drugs that come in prefilled glass syringes.
Copyright ©2004 Medical Product Manufacturing News
You May Also Like

.png?width=300&auto=webp&quality=80&disable=upscale)