Charge-Neutral Compound Optimizes Dosage for Asthma Inhaler
Use of an antistatic polymer prevented static buildup from compromising patient care in a valve holding chamber
September 12, 2009
Originally Published MPMN September 2009
ENGINEERING SOLUTIONS
Charge-Neutral Compound Optimizes Dosage for Asthma Inhaler
Use of an antistatic polymer prevented static buildup from compromising patient care in a valve holding chamber
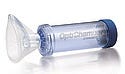
Click to enlarge |
The use of an antistatic compound with high optical clarity helped to improve the design of an asthma product. |
Often employed by such demographics as children and the elderly, valve holding chambers (VHCs) are designed for asthma patients who have difficultly coordinating their inspiration with the activation of an aerosol inhaler. But while VHCs assist patients in properly administering medication, many of them are vulnerable to static buildup, which can hinder administration of consistent dosages. And, in the case of drug-delivery devices, ensuring that patients receive consistent dosages is essential to successful treatment.
Desiring to improve drug delivery and patient care for its OptiChamber Advantage II VHC, Philips Respironics (Murrysville, PA; www.respironics.com) sought to overcome this common design flaw. To do so, Philips partnered with custom compounder RTP Co. (Winona, MN; www.rtpcompany.com), with whom it had a long-standing relationship, to develop a charge-neutral material for the product.
Use of a charge-neutral material would eliminate the product’s primary drawback: VHC housings can attract and hold residual amounts of medication due to static buildup. Because of this problem, the products also require the added inconvenience of washing and air-drying to reduce static prior to inhaler use. Moreover, the need for static-free VHCs has increased recently in response to a new class of inhalers on the market that have replaced chlorofluorocarbon (CFC) propellants with hydrofluoroalkane (HFA) ones.
“The use of a charge-neutral material in our VHC was vital to meeting the needs of healthcare providers and their patients through improved design,” says Robert Koshinskie Sr., product manager for Philips Respironics.
In order to achieve the neutral charge for the chamber, Respironics worked closely with RTP engineers to find a conductive material that would eliminate static buildup. Furthermore, it was essential to maintain physical properties, most notably the high optical clarity desired for a nearly water-clear appearance to aid in the product’s operation.
Research conducted by RTP in partnership with Philips Respironics showed that the substitution of a PermaStat 600-series all-polymeric, permanently antistatic ABS compound for the original material would greatly improve drug-delivery consistency. Such consistency results in better treatment for patients using the OptiChamber Advantage.
“ABS materials in general have good impact properties, which make them ideal for use on handheld items like inhalers,” notes Josh Blackmore, global healthcare manager, RTP. “Additionally, RTP Co.’s PermaStat ABS family of materials has been proven to pass biocompatibility, leachability, and drug-interaction testing, which makes them suitable for use in the drug flow path of inhalers.” The material’s low resistivity also provided Respironics engineers with just the right amount of static dissipation to optimize the chamber’s functionality.
“By addressing static attraction, the product offers consistent and reliable delivery on first use out of package, whether washed or unwashed,” states Koshinskie. “This material gave us a great balance of low resistivity, high clarity, and strength, which was key to the success of our product.”.
For more engineering solution stories, go to devicelink.com/mpmn/engineering
Copyright ©2009 Medical Product Manufacturing News
You May Also Like