Changing Healthcare Delivery through New Technologies
Originally Published MDDI April 2006MDEA 2006 These winners not only upgraded existing products, but revolutionized them. Sherrie Conroy
April 1, 2006
MDEA 2006
When a new device comes on the market, it is often a simple upgrade of something already available. It's not only difficult—it can be quite expensive to develop a product that is truly revolutionary. But there are those few devices that come along that may just change the way doctors and hospitals deliver healthcare. And, as expected, these devices are, by nature, award winners. A few of these potentially disruptive technologies are highlighted here.
|
The OmniPod delivers insulin at preprogrammed rates. The system eliminates wires between the pump and the controller. |
OmniPod
The OmniPod insulin management system, manufactured by Insulet Corp. (Bedford, MA), delivers insulin at preprogrammed rates to people with diabetes. The system includes a handheld personal diabetes manager that communicates wirelessly to a three-day wearable insulin-delivery device.
“There is a lot of patient involvement in the management of diabetes,” explains John Garibotto, vice president of engineering for Insulet. “When the patient is in the doctor's office, the doctor determines whether the patient is technically savvy enough to handle the currently available pumps. “There are lots of mistakes that could happen,” he says. “For example, a patient can over infuse and become hypoglycemic.”
With the OmniPod, insulin enters the body through a soft cannula that is inserted into the subcutaneous tissue. The system uses a handheld diabetes manager that communicates wirelessly to the OmniPod. Because it has no tubing, patients sleep more comfortably.
“With tubing sets, it is difficult to inject the needle at the right angle into the subcutaneous layer. Patients often insert too shallow or too deep (intramuscular),” he says. “With the pod, the cannula is placed on the body and, through wireless automation, the needle and tubing are inserted at a 45Þ angle subcutaneous every time. There are no sharps because the needle is retracted into the device.”
The system's automated cannula insertion system is designed to reduce physical discomfort resulting from insertion errors because it eliminates variability in insertion angle and depth, says Garibotto. “The patient never sees the needle, and there are no sharps to dispose of after use,” he says. The handheld portion programs the pod with delivery instructions and monitors the pod's operation. The handheld device contains the glucose meter and automatically stores patient records.
“What was very appealing about this product was the elimination of user action in the cannula insertion step,” says MDEA juror Craig Jackson, president and chief technical officer of Hemosage Diagnostic Corp. (San Diego).
According to Garibotto, conventional insulin pumps have hundreds of components. By separating the user interface and focusing on minimizing part count, Insulet reduced the number of components in the pod to 40 parts.
The OmniPod is designed to bring the therapy to a larger patient population. “From the perspective of intuitiveness and ease of use,” says Garibotto, “we tried to see why insulin pumps were not as prevalent as they should be as a therapy to treat diabetes.” The OmniPod has the same overall cost over a four-year period as conventional insulin pump therapy; however, it doesn't have the typical large up-front costs. Costs are distributed over the life of the product. “Insulin pump therapy remains out of reach for many diabetes patients who would most benefit from pump therapy,” explains Garibotto. In addition to cost, another factor that rules out some patients is their inability to see the small screen of existing pumps. Insulet made the user interface a separate handheld wireless device. “It's much friendlier and explains instructions in full sentences,” says Garibotto.
Jackson points to the elimination of wires between the pump and the controller as an important part of the device's design. “Added control is possible using the integrated glucose monitor, and it provides more-appropriate insulin dosage, that is, better glycemic control,” he says.
“We want to make pumps available to a wider audience,” says Garibotto. “By designing the separate interface, the pump is much easier to use. Doctors can present this pump to patients they may have previously ruled out,” says Garibotto. Feedback from patients who have used the device has been overwhelmingly positive, he says.
From a technical standpoint, Garibotto notes that one of the company's key decisions was the selection of the motor. “We used a nitinol wire as the actuator to drive the insulin. Current pumps use a dc motor. The wire meant a smaller form factor and less weight,” he says. “It also cost only 25 cents compared with a dc motor, which costs around $100.”
The company did get one surprise. “When we started, we were interested in converting existing pump users. We have found, however, that more than 70% of our users are coming from conventional insulin patients—those who inject insulin. Existing pump patients have been slower to convert. This is one of our most interesting findings.” Garibotto attributes this outcome to the way that insulin pumps are covered by health insurance and the expensive investment diabetics make in their current pumps.
The OmniPod is the culmination of five years of development. Insulet is a start-up company that was founded to develop this insulin management system.
Nucleus Freedom
The Nucleus Freedom system, developed by Cochlear Ltd. (Lane Cove, NSW, Australia) restores hearing to people with profound hearing loss. Sounds picked up by a processor behind the ear are transmitted by radio to the implant. The implanted electrodes stimulate the cochlear nerve, and this stimulation of the nerve is perceived as sound.
|
The Nucleus Freedom system restores hearing by using implanted electrodes to stimulate the cochlear nerve. |
The Nucleus Freedom is the fourth-generation of cochlear implant technology developed by the company. Modern cochlear implants enable recipients to hear well in quiet situations, but it can be difficult for them to hear in environments like restaurants because of the ambient noise. According to the company, the Nucleus Freedom is the first implant system to use beam-forming technology, which is specifically designed to function well in noisy situations. The technology detects and eliminates noise from any direction other than the speaker straight in front of the user.
“More than 7000 people have seen a dramatic change in their lives since we launched the product last year, and 20% of them are under 3 years old,” says Robert Southwood, program manager for product development for Cochlear Ltd. “A child who is born deaf and receives a Nucleus Freedom system can attend mainstream school and can have life opportunities similar to those of a child with normal hearing,” says Southwood. “At the other end of the spectrum are the 20% of Freedom users over the age of 65, many of whom are enjoying sounds that they haven't heard for decades or who are able to communicate with their grandchildren for the first time,” he says.
“Studies have shown that of all medical interventions, the cochlear implant system is second only to neonatal intensive care in terms of the quality of life improvement per dollar spent,” Garibotto says.
According to the company, a design goal for the device was to prevent ingress of liquid and contaminants, and implant reliability was paramount. A speech processor chip consumes 50% less power than the digital signal processor (DSP) application-specific integrated circuit (ASIC) in the previous generation system.
“Cochlear implants can be expensive to run due to the cost of the batteries used by the external speech processor,” explains Southwood. “Power consumption is at the forefront of our designers' minds in order to reduce the cost of batteries for patients and to reduce the inconvenience of having to frequently change batteries,” he says. Because the newer speech detection algorithms can also require more power, he says that saving power in the DSP ASIC gives more patients access to better performance.
“With Nucleus Freedom, most patients can run advanced algorithms for three days before having to change their batteries. With some older products, patients had to change their batteries twice in one day.”
This new system comprises four components: an electronic module that is surgically implanted under the skin behind the ear (including an electrode that is inserted inside the cochlea cavity); a speech processor worn externally behind the ear like a hearing aid that converts environmental sounds, such as speech, into electrical stimulation patterns transmitted to the implant over a radio-frequency link; software used by audiologists to test the performance of the implant and speech processor; and a small piece of hardware connecting the audiologist's computer to the patient's speech processor.
The company conducted extensive market research in many countries before starting to design the Nucleus Freedom. “We wanted to make sure we really heard and understood the voices of all of our customers,” says Southwood. “For example, they told us that conventional cochlear implants work well in quiet surroundings but not so well in background noise, say at school or in a restaurant, so this became a high priority for us in the development of the beam algorithm. They told us that children wanted to be able to wear their processor in the rain, or to run through a sprinkler, or to play sports in humid conditions, so we designed the external parts to be water resistant,” he says.
Southwood says Cochlear implemented dozens of features as a direct result of carefully listening to customers and to clinical professionals. “We also conducted a lot of concept testing sessions, and we did extensive prototyping. We conducted an exhaustive clinical trial to make sure we got the features just right,” says Southwood. “Even now, a year after launch, we are monitoring customer experience and making fine-tune adjustments and adding some major new improvements to the Nucleus Freedom system.”
|
The IDI-MRSA assay can provide definitive and fast results, allowing earlier treatment. |
IDI-MRSA Assay
The IDI-MRSA assay, developed by GeneOhm Sciences Inc. (San Diego), is a qualitative in vitro diagnostic test for the direct detection of nasal colonization by methicillin-resistant Staphylococcus aureus (MRSA) to help prevent and control MRSA infections in healthcare settings. In March, Becton Dickinson acquired GeneOhm, which will now be known as BD Diagnostics– GeneOhm business unit.
Infection control programs that use active surveillance to identify patients and strict application of barrier precautions for patients colonized or infected with MRSA have shown success in controlling MRSA. The company's IDI-MRSA assay provides results in just two hours of lab time compared with the 16–72 hours required for a conventional culture.
According to the company, such definitive, rapid results combined with earlier treatment intervention can significantly improve patient outcomes, prevent outbreaks, and control hospital costs. Without active surveillance testing for MRSA, most colonized patients go undetected.
“MRSA is increasing at an alarming rate in hospitals, carrying a heavy burden of mortality and morbidity. In the United States alone, CDC estimates MRSA accounts for 2 million infections, 8 million excess hospital days, and more than 90,000 deaths per year,” explains Peter Klemm, president of BD Diagnostics–GeneOhm. “In fact, it is ranked as the fifth-leading cause of death in hospitals.”
“The key is time—sooner is better,” says MDEA juror Jackson. “The sooner identification is made, the sooner appropriate action can be taken. The action is treatment of the infected patient who is at the greatest risk simply because he or she is sick. Many may be immunocompromised,” he says. “Even more effective is the identification of hospital employees who are carriers and then eliminating the opportunity for them to inadvertently infect others, particularly patients,” says Jackson.
Klemm says that hospitals can now actively prevent infections, rather than merely reacting to control them. “Identifying the reservoir of MRSA carriers enables better infection control processes, leading to reductions in the transmission and infection rates of MRSA,” notes Klemm. “Early screening of patients for MRSA nasal carriage to identify those patients that require isolation can be a pivotal part of an effective infection control program.”
According to the company, current techniques require one or more culture steps. They require the isolation of pure colonies followed by either oxacillin susceptibility testing, detection of the medA gene, or detection of the penicillin binding protein (PBP 2a) encoded by the mecA gene or an 18–24-hour incubation on a specialized agar plate. The time to definitive resolution of MRSA carrier status takes a minimum of 16 hours.
“Providing the absolute minimal turnaround time was a critical design goal, combined with achieving performance equal to or exceeding the gold standard of culture,” says Klemm. “Another significant aspect of the turnaround time is tied to the negative predictive value of 98%.” He says that knowing within hours that a patient is not colonized with MRSA enables hospitals to avoid the costs and complications of isolating patients under contact precautions while they wait for up to two additional days for a definitive test result.
“By enabling hospitals to identify patients colonized with MRSA within hours of admission, or even prior to admission, use of this rapid MRSA test means that hospitals no longer have to accept these infections as a cost of doing business,” says Klemm. “Armed with the early, actionable information provided by this rapid test, hospitals can employ targeted interventions to prevent medical complications, reduce the spread of infection, shorten hospital stays, reduce medical costs, and improve patient outcomes.”
Many healthcare institutions have implemented active surveillance screening and search-and-destroy policies that use a zero tolerance approach to multi-drug-resistant organisms like MRSA, says Klemm. “As the prevalence of MRSA, as well as other multi-drug-resistant organisms and healthcare-associated infections, such as Vancomycin-resistant Enterococcus (VRE) and Clostridium difficile, continue to increase, it is expected that hospitals will be driven to adopt best practice protocols that are demonstrated to reduce infection rates.”
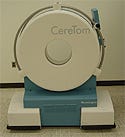
CereTom Mobile CT Scanner
The CereTom Mobile CT (computed tomography) Scanner, developed by Neurologia (Danvers, MA), is a compact, lightweight, mobile, high-speed, battery and line-powered multislice CT scanner. It is optimized for scanning anatomy that can be imaged in the 25-cm field of view, primarily head and neck. The system generates up to eight slices per revolution, using the system's Modular Multirow Detector (MMD).
|
The CereTom Mobile CT Scanner generates up to eight slices per revolution and transmits the images wirelessly. |
“The eight-slice capability means that you do not sacrifice quality to get an extremely valuable asset that can be transported to a critical patient,” says juror Jeff Butler, principal engineer for Sysmed Enterprises (Richardson, TX). “ICU patients are switched from bedside monitoring to a portable monitoring system and wheeled down hallways to the CT scanner accompanied by nurses or other medical professionals who generally have other responsibilities.”
The CereTom's wireless image transfer system enables clinicians to process examinations wherever a patient is located. The scanner is designed to provide high-quality imaging anywhere it is needed. Any clinician can use the scanner to obtain noncontrast CT, CT angiography, and CT perfusion studies of the head and neck in as little as two minutes. The Silhouette scan board, an accessory to the scanner, converts gurneys, ICU beds, operating room tables, or hospital beds into the scanning platform.
The bed remains stationary in a patient's room or in ICU. The patient never moves; only the scanner moves. Trauma to patients is greatly reduced or eliminated by minimizing the need to transport for CT scanning. According to the company, the CereTom has very low scatter radiation, which makes it safe to operate anywhere in the hospital, without the need for a shielded room.
The scanner provides three major benefits to patients; lower dose during scanning, elimination of the need for transport, and faster scan times. The CereTom scanner delivers less than one-third the radiation than that of a standard CT scanner while delivering the same image quality. The patient is exposed to a lower dose, making it a safer diagnostic imaging alternative. By converting the patient bed into the scanning platform, the scanner eliminates the need to transport critically ill patients for head and neck imaging. Eliminating transport greatly reduces the risk to patients and thus improves patient outcomes.
“The scanner is a compact, lightweight, high-speed mobile battery-powered multislice CT on wheels,” says juror Matthew Weinger. Weinger is professor of anesthesiology, biomedical informatics, and medical education at Vanderbilt University School of Medicine (Nashville, TN). “Preprogrammed protocols are automated into one touch. It can be operated with minimal training,” he says.
The major benefit of the CereTom is that clinicians can move the point of care to the patient. According to the company, the CereTom is the first major upgrade to the delivery of CT scans since these scanners were first put into mobile trailers in the early 1990s. As a result, more patients will have the opportunity to be scanned in a safer and more efficient manner. “This is a very interesting product,” says Butler.
|
The Image Guided Implantology system guides dental implantation surgical instruments with a CT-based surgical plan. |
Image Guided Implantology System
The Image Guided Implantology system, manufactured by DenX Advanced Dental Systems Ltd. (Jerusalem, Israel), is a computerized navigational system that assists in the preoperative and intraoperative phases of dental implantation surgery, accurately guiding surgical instruments according to a CT-based presurgical plan.
The system provides assistance in both the planning (preoperative) and the surgical (intraoperative) phases of dental implantation surgery. In the preoperative phase, the system enables the surgeon to accurately evaluate the alveolar bone and the critical anatomical structures, as displayed on a patient's dental CT scan. Consequently, the system enables the surgeon to create an accurate digital preoperative plan of implants' positions, based on the CT imaging. The planned positions are correlated to the anatomical structures and to the patient's individual occlusion.
In the intraoperative phase, the system guides the surgeon to place the implants exactly as planned and warns the surgeon of significant deviations from the plan. According to the company, this reduces risk of failure and damage to adjacent structures. The combination of the pre- and intraoperative steps ensures a quality final restoration. The optics-based navigational system uses an infrared camera sensor and light-emitting diodes to monitor objects in a confined space. The system includes a tracker attached to a standard dental handpiece and a patient tracker attached to the patient's jaw through a pole screwed to a horseshoe.
One of the key benefits of the system is that it allows less-experienced practitioners to place implants with confidence, the company says. An important consideration at all stages of design was the need to simplify the work flow and overhead associated with the system. The system promotes a more controlled protocol for implant placement, which increases safety and contributes greatly to achieving optimal restoration and implant placement results. This is particularly evident in the edentulous (toothless) patients. The company has developed a unique hardware kit to address the needs of these patients.
“Many techniques and procedures in medicine are more art than science, or at least require significant art or learned techniques,” says juror Butler. “Products like this one shorten the learning curve for the art of implanting and introduce more precision. To see—really see—what the physician is doing can be quite revolutionary,” he says.
Copyright ©2006 Medical Device & Diagnostic Industry
About the Author(s)
You May Also Like