CDRH Begins Reform of Postmarket Monitoring
Originally Published MDDI March 2006NewsTrends Erik Swain
March 1, 2006
NewsTrends
|
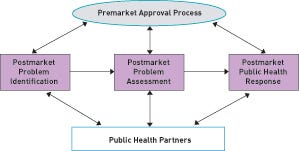
Figure 1. The CDRH postmarket safety framework operates through three integrated areas: postmarket problem identification, postmarket problem assessment, and postmarket public health response. |
FDA and industry have taken the first steps toward overhauling the agency's monitoring of the safety of medical devices once they reach the market.
The Postmarket Transformation Initiative was announced in January. It aims to identify, analyze, and respond to problems in the field more quickly. It also intends to alert the public faster of potential problems concerning devices.
In February, CDRH and AdvaMed held a joint workshop, the first of several to help put the initiative into practice. Its main purpose was to “identify potential gaps in coverage,” says Mark Brager, AdvaMed's director of communications.
The agency's postmarket surveillance practices had come under intense criticism in the past year. At first, the focus of the complaints was on drugs. But attention shifted to CDRH after problems with implantable cardioverter-defibrillators from Indianapolis-based Guidant Corp. were revealed.
At the core of the initiative are the agency's efforts to devise an electronic reporting system for adverse events and to improve device information in patient records. CDRH will also reform how it collaborates internally on postmarket safety issues. This reform began last year when CDRH shifted responsibility for monitoring postmarket clinical studies to the Office of Surveillance and Biometrics.
CDRH and AdvaMed took away three major points from the February meeting, says Brager.
The first was identifying areas where FDA and industry could use enhanced training. These were quality systems, postapproval studies, recall procedures, and reporting of adverse events.
The second was agreeing to set up joint workshops on specific topics. These workshops will cover recalls, annual reports, condition-of-approval studies, and unique device identifiers. As part of the initiative, CDRH promised to come up with unique ways to identify devices, so that the agency can more easily recognize problems mentioned in reports. Brager says that AdvaMed is uncertain about that proposal, however. “We are always concerned about any regulation or legislation that has a ‘one size fits all' solution to a problem that we are not sure exists,” he says. “It's important that the discussion involve the hospital and physician communities, because they are the ones that have to identify the devices.”
The third point was setting up more-formal workshops on topics such as risk management and human factors.
“We have established clear goals to promptly identify and analyze adverse events related to devices once they are on the market and to alert device users of potential risks,” said CDRH director Daniel Schultz in an agency press release. “Specifically, we are strengthening the requirements for industry-sponsored studies once their devices are on the market; improving our targeted surveillance systems to identify adverse events; enhancing our risk-based approach to inspecting manufacturing sites, including implementing third-party inspections; improving our communication of risk-benefit information to the public and healthcare providers; and increasing use of automated information systems.”
The initiative came about after a yearlong investigation of the tools CDRH uses to monitor postmarket safety. Senior FDA management and several consultants have been tapped to guide the implementation of the initiative.
“We are committed to work with the agency to identify and review any problems that come to light,” says Brager. “But the bottom line is that any changes that are made must be things that can be shown to improve the public health. Nice ideas that won't improve the public health are not the purpose here.”
Mark Leahey, executive director of the Medical Device Manufacturers Association, agrees. “We want to make sure these issues are addressed, but not in a way that makes the requirements overly burdensome, so companies are not able to transmit information efficiently and effectively,” he says. “We want to get information to the agency more quickly without reinventing the wheel. And if they need more information, we need to figure out how to do that without compromising proprietary situations.”
More information about the initiative can be found at www.fda.gov/cdrh/postmarket/mdpi.html.
Copyright ©2006 Medical Device & Diagnostic Industry
About the Author(s)
You May Also Like