009 WINNING PRODUCTS AND SUPPLIERS
Critical-Care and Emergency Medicine Products
April 1, 2009
MDEA 2009
 Lifepak 15 monitor-defibrillator, manufactured and submitted by Physio-Control Inc. (Redmond, WA). The Lifepak 15 monitor-defibrillator is used in medical emergencies to provide defibrillation and a range of monitoring functions, so that emergency medical services and hospital personnel can provide timely and appropriate patient care.
Lifepak 15 monitor-defibrillator, manufactured and submitted by Physio-Control Inc. (Redmond, WA). The Lifepak 15 monitor-defibrillator is used in medical emergencies to provide defibrillation and a range of monitoring functions, so that emergency medical services and hospital personnel can provide timely and appropriate patient care.
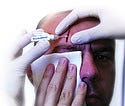 LiquiBand Optima wound-closure adhesive, manufactured and submitted by Medlogic Global Ltd. (Plympton, Plymouth, Devon, UK). LiquiBand Optima is an advanced cyanoacrylate skin adhesive and applicator. Its no-sting, fast-set formulation is optimized for safe, quick, and easy use, and application to close minor wounds in the emergency room.
LiquiBand Optima wound-closure adhesive, manufactured and submitted by Medlogic Global Ltd. (Plympton, Plymouth, Devon, UK). LiquiBand Optima is an advanced cyanoacrylate skin adhesive and applicator. Its no-sting, fast-set formulation is optimized for safe, quick, and easy use, and application to close minor wounds in the emergency room. 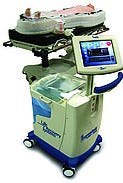 ThermoSuit body-cooling system, manufactured by Life Recovery Systems (Waldwick, NJ). Entry submitted by Ximedica LLC (Providence, RI). ThermoSuit cools the core temperature of cardiac arrest, stroke, or heart attack victims to 33°C in 20 minutes or less. When cooled, vital organs operate more slowly and require less oxygen, reducing the chance of brain or heart damage.
ThermoSuit body-cooling system, manufactured by Life Recovery Systems (Waldwick, NJ). Entry submitted by Ximedica LLC (Providence, RI). ThermoSuit cools the core temperature of cardiac arrest, stroke, or heart attack victims to 33°C in 20 minutes or less. When cooled, vital organs operate more slowly and require less oxygen, reducing the chance of brain or heart damage.
Supply and design credit to Ximedica LLC (Providence, RI), Nexcore Technologies Inc. (Waldwick, NJ), and Dielectrics Inc. (Chicopee, MA).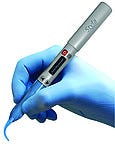 Styla MicroLaser advanced dental laser system, manufactured by Zap Lasers (Pleasant Hill, CA). Entry submitted by KRT Marketing (Lafayette, CA). The Styla is dentistry's first microlaser, enabling dentists to perform soft-tissue procedures with little or no patient anesthetic, recession, bleeding, or pain. The unit is 6.9 in. long and weighs just 1.9 oz.
Styla MicroLaser advanced dental laser system, manufactured by Zap Lasers (Pleasant Hill, CA). Entry submitted by KRT Marketing (Lafayette, CA). The Styla is dentistry's first microlaser, enabling dentists to perform soft-tissue procedures with little or no patient anesthetic, recession, bleeding, or pain. The unit is 6.9 in. long and weighs just 1.9 oz.  UniDose glucose meter check control, manufactured and submitted by Bionostics (Devens, MA). Glucose meter check control in UniDose provides a simple-to-use, reliable, and robust container to provide and present a single drop of quality-control solution to verify the performance of a blood glucose test system.
UniDose glucose meter check control, manufactured and submitted by Bionostics (Devens, MA). Glucose meter check control in UniDose provides a simple-to-use, reliable, and robust container to provide and present a single drop of quality-control solution to verify the performance of a blood glucose test system.  Envision E700 wound surface, manufactured and submitted by Hill-Rom Co. (Charleston, SC). The Envision E700 wound surface addresses five key factors affecting pressure ulcer healing: pressure, shear, friction, patient immobility, and microclimate management. It can be reconfigured for any type of sleep deck: step-deck, V-deck, or flat deck.
Envision E700 wound surface, manufactured and submitted by Hill-Rom Co. (Charleston, SC). The Envision E700 wound surface addresses five key factors affecting pressure ulcer healing: pressure, shear, friction, patient immobility, and microclimate management. It can be reconfigured for any type of sleep deck: step-deck, V-deck, or flat deck.  Medigenic infection control keyboard, manufactured and submitted by Esterline Advanced Input Systems (Coeur d'Alene, ID). The Medigenic infection control keyboard addresses studies showing hospital keyboards to be a source of bacterial cross-contamination. The Medigenic keyboard helps monitor its own cleaning status and allows for high-speed data entry and quick cleaning.
Medigenic infection control keyboard, manufactured and submitted by Esterline Advanced Input Systems (Coeur d'Alene, ID). The Medigenic infection control keyboard addresses studies showing hospital keyboards to be a source of bacterial cross-contamination. The Medigenic keyboard helps monitor its own cleaning status and allows for high-speed data entry and quick cleaning. 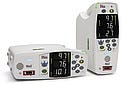 Rad-87 pulse CO-oximeter, manufactured and submitted by Masimo (Irvine, CA). The Rad-87 is a pulse CO-oximeter with optional built-in 802.11a/b/g radio for bidirectional wireless communication with a remote monitoring and clinician notification system. It allows easy activation of features with a single touch.
Rad-87 pulse CO-oximeter, manufactured and submitted by Masimo (Irvine, CA). The Rad-87 is a pulse CO-oximeter with optional built-in 802.11a/b/g radio for bidirectional wireless communication with a remote monitoring and clinician notification system. It allows easy activation of features with a single touch.  S3 medical-surgical hospital bed, manufactured and submitted by Stryker Medical (Portage, MI). The S3 medical-surgical hospital bed is a patient support platform incorporating many safety, patient-centric, and nursing-efficiency features that are simple to use and reliable.
S3 medical-surgical hospital bed, manufactured and submitted by Stryker Medical (Portage, MI). The S3 medical-surgical hospital bed is a patient support platform incorporating many safety, patient-centric, and nursing-efficiency features that are simple to use and reliable.
Supply and design credit to Hilco Technologies (Grand Rapids, MI), Advantage Sintered Metals Inc. (Battle Creek, MI), Eimo Technologies Inc. (Vicksburg, MI), Quality Tool and Stamping (Muskegon Heights, MI), and Cascade DieCasting/Great Lakes (Sparta, MI).  InterFuse interbody fusion device, manufactured and submitted by Vertebral Technologies Inc. (Minnetonka, MN). The InterFuse device is an interbody fusion device for the spine that provides a customized fit for each patient using a minimally invasive approach. Its unique rail-and-slot design locks up to six small modules together into a solid, large construct.
InterFuse interbody fusion device, manufactured and submitted by Vertebral Technologies Inc. (Minnetonka, MN). The InterFuse device is an interbody fusion device for the spine that provides a customized fit for each patient using a minimally invasive approach. Its unique rail-and-slot design locks up to six small modules together into a solid, large construct.
Supply and design credit to Invibio Inc. (Woodbury, MN), Minnesota Rubber and Plastics (Centerville, MN), and Rochester Medical Implants (Rochester, IN). iUni and iDuo (unicompartmental and bicompartmental) knee resurfacing devices, manufactured by ConforMIS Inc. (Burlington, MA). Entry submitted by Racepoint Group (Waltham, MA). The iUni and iDuo (unicompartmental and bicompartmental) are personalized knee resurfacing implants that provide patients and surgeons with an early intervention option for osteoarthritis of the knee.
iUni and iDuo (unicompartmental and bicompartmental) knee resurfacing devices, manufactured by ConforMIS Inc. (Burlington, MA). Entry submitted by Racepoint Group (Waltham, MA). The iUni and iDuo (unicompartmental and bicompartmental) are personalized knee resurfacing implants that provide patients and surgeons with an early intervention option for osteoarthritis of the knee.  Merlin.net patient care network (PCN), manufactured and submitted by St. Jude Medical Inc. CRMD (Sylmar, CA). Merlin.net PCN is an integrated system enabling telemetry transmissions from patients' implanted medical devices from home. The Internet portal and real-time alert management extends the reach of healthcare from the clinic to the home.
Merlin.net patient care network (PCN), manufactured and submitted by St. Jude Medical Inc. CRMD (Sylmar, CA). Merlin.net PCN is an integrated system enabling telemetry transmissions from patients' implanted medical devices from home. The Internet portal and real-time alert management extends the reach of healthcare from the clinic to the home.
Supply and design credit to Insight Product Development (Chicago), IBM (Glendale, CA), and Plexus Services Corp. (Neenah, WI). Bactec FX microbial detection system, manufactured by Becton, Dickinson and Co. (Sparks, MD). Entry submitted by Bresslergroup Inc. (Philadelphia). The Bactec FX is a microbial detection system used for growing and detecting microorganisms in clinical specimens. The product's ergonomically optimized design offers an efficient work flow and high-capacity output with a small footprint.
Bactec FX microbial detection system, manufactured by Becton, Dickinson and Co. (Sparks, MD). Entry submitted by Bresslergroup Inc. (Philadelphia). The Bactec FX is a microbial detection system used for growing and detecting microorganisms in clinical specimens. The product's ergonomically optimized design offers an efficient work flow and high-capacity output with a small footprint.
Supply and design credit to Bresslergroup Inc. (Philadelphia), Crescent Industries Inc. (New Freedom, PA), and CW Thomas Inc. (Philadelphia). eSensor XT-8 system for warfarin sensitivity testing, manufactured and submitted by Osmetech Molecular Diagnostics (Pasadena, CA). The eSensor XT-8 system for warfarin sensitivity testing detects multiple genetic variations that identify patients at risk for warfarin sensitivity and thus allows estimation of the optimal and safe warfarin dose.
eSensor XT-8 system for warfarin sensitivity testing, manufactured and submitted by Osmetech Molecular Diagnostics (Pasadena, CA). The eSensor XT-8 system for warfarin sensitivity testing detects multiple genetic variations that identify patients at risk for warfarin sensitivity and thus allows estimation of the optimal and safe warfarin dose.
Supply and design credit to Aubrey Group Inc. (Irvine, CA), and Aline Inc. (Redondo Beach, CA). Benchmark Ultra immunohistochemistry and in situ hybridization staining instrument, manufactured and submitted by Ventana Medical Systems Inc. (Tucson, AZ). The Benchmark Ultra is an immunohistochemistry and in situ hybridization staining instrument with advanced features that improve usability, streamline laboratory work flow, and result in faster reporting of patient test results.
Benchmark Ultra immunohistochemistry and in situ hybridization staining instrument, manufactured and submitted by Ventana Medical Systems Inc. (Tucson, AZ). The Benchmark Ultra is an immunohistochemistry and in situ hybridization staining instrument with advanced features that improve usability, streamline laboratory work flow, and result in faster reporting of patient test results.
Supply and design credit to Flextronics Inc. (Jesús Maria, Mexico), SMC Corp. of America (Tempe, AZ), Baymar Solutions (Lutz, FL), and Intuition Design Inc. (Chesapeake City, MD). INRatio2 blood coagulation monitor, manufactured by Hemosense, Inverness Medical (Hayward, CA). Entry submitted by IDEO (Palo Alto, CA). The INRatio2 enables patients to test blood coagulation parameters—prothrombin time and international normalized ratio—at home. Results are obtained in one minute using a single drop of blood, thereby improving care and increasing patient convenience.
INRatio2 blood coagulation monitor, manufactured by Hemosense, Inverness Medical (Hayward, CA). Entry submitted by IDEO (Palo Alto, CA). The INRatio2 enables patients to test blood coagulation parameters—prothrombin time and international normalized ratio—at home. Results are obtained in one minute using a single drop of blood, thereby improving care and increasing patient convenience.
Supply and design credit to IDEO (Palo Alto, CA). Spectra MRSA chromogenic plated medium, manufactured and submitted by Thermo Fisher Scientific (Lenexa, KS). Spectra MRSA is a chromogenic plated medium intended for the primary isolation of MRSA from nasal swabs with conclusive results in 24 hours. It provides a reliable, economical solution to control and reduce the spread of MRSA.
Spectra MRSA chromogenic plated medium, manufactured and submitted by Thermo Fisher Scientific (Lenexa, KS). Spectra MRSA is a chromogenic plated medium intended for the primary isolation of MRSA from nasal swabs with conclusive results in 24 hours. It provides a reliable, economical solution to control and reduce the spread of MRSA.  TearLab osmolarity system, manufactured by TearLab Corp. (San Diego). Entry submitted by Invetech (Mt. Waverley, VIC, Australia). The TearLab osmolarity system is a point-of-care in vitro lab-on-a-chip diagnostic for eyecare enabling analysis of less than 50 nanoliters of tear fluid, making tear collection and analysis safe, simple, and rapid.
TearLab osmolarity system, manufactured by TearLab Corp. (San Diego). Entry submitted by Invetech (Mt. Waverley, VIC, Australia). The TearLab osmolarity system is a point-of-care in vitro lab-on-a-chip diagnostic for eyecare enabling analysis of less than 50 nanoliters of tear fluid, making tear collection and analysis safe, simple, and rapid.
Supply and design credit to Invetech (Mt. Waverley, VIC, Australia). True2go portable glucose monitor, manufactured and submitted by Home Diagnostics Inc. (Fort Lauderdale, FL). True2go is the world's smallest glucose meter. Suited for on-the-go use, the reusable meter attaches to the strip vial, gives results in as little as 4 seconds from a 0.5-μl sample, and self-calibrates using coding technology embedded on each test strip.
True2go portable glucose monitor, manufactured and submitted by Home Diagnostics Inc. (Fort Lauderdale, FL). True2go is the world's smallest glucose meter. Suited for on-the-go use, the reusable meter attaches to the strip vial, gives results in as little as 4 seconds from a 0.5-μl sample, and self-calibrates using coding technology embedded on each test strip.
Supply and design credit to Integrated Engineering Technologies (Tacoma, WA), Texas Instruments Inc. (Maitland, FL), and CSP Technologies (Amsterdam, NY). Vitros 5600 integrated system, manufactured and submitted by Ortho Clinical Diagnostics (Rochester, NY). The Vitros 5600 integrated system is a high-capacity clinical laboratory system designed to integrate clinical chemistry and immunoassay testing into a single instrument.
Vitros 5600 integrated system, manufactured and submitted by Ortho Clinical Diagnostics (Rochester, NY). The Vitros 5600 integrated system is a high-capacity clinical laboratory system designed to integrate clinical chemistry and immunoassay testing into a single instrument.
Supply and design credit to Priority Designs (Columbus, OH).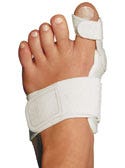 Bunion Aid splint, manufactured and submitted by Alpha Orthotics Corp. (Tiburon, CA). The Bunion Aid hinged splint is designed to correct mild to moderate hallux valgus mal-positioning to normal values. The day/night splint consists of a medical-grade plastic hinge and antibacterial metatarsal toe straps and pads.
Bunion Aid splint, manufactured and submitted by Alpha Orthotics Corp. (Tiburon, CA). The Bunion Aid hinged splint is designed to correct mild to moderate hallux valgus mal-positioning to normal values. The day/night splint consists of a medical-grade plastic hinge and antibacterial metatarsal toe straps and pads.
Supply and design credit to Hallufix AG (Munich), Office of Dr. Klaus A. Milachowski (Munich), and Fraunhofer-Gesellschaft zur Förderung (Munich). Accu-Right CT marker device, manufactured and submitted by Accu-Right Correct Side Solutions LLC (Los Angeles). The Accu-Right CT marker device is a safety innovation that labels the right side in computed tomography images with a capital letter R to distinguish it from the left side. This recyclable CT accessory may reduce wrong-side surgery.
Accu-Right CT marker device, manufactured and submitted by Accu-Right Correct Side Solutions LLC (Los Angeles). The Accu-Right CT marker device is a safety innovation that labels the right side in computed tomography images with a capital letter R to distinguish it from the left side. This recyclable CT accessory may reduce wrong-side surgery.
Supply and design credit to ProAction Products (Van Nuys, CA), HLB (Chicago), VIP Rubber and Plastic Products Inc. (La Habra, CA).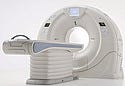 Aquilion One dynamic volume computed tomography system, manufactured and submitted by Toshiba America Medical Systems Inc. (Tustin, CA). The Aquilion One dynamic volume computed tomography system reduces diagnosis time from hours or days to minutes. It can image up to 16 cm in a single rotation and show dynamic movement. Its comprehensive exam reduces the need for other tests.
Aquilion One dynamic volume computed tomography system, manufactured and submitted by Toshiba America Medical Systems Inc. (Tustin, CA). The Aquilion One dynamic volume computed tomography system reduces diagnosis time from hours or days to minutes. It can image up to 16 cm in a single rotation and show dynamic movement. Its comprehensive exam reduces the need for other tests. 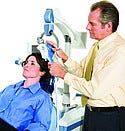 NeuroStar TMS Therapy system, manufactured and submitted by Neuronetics Inc. (Malvern, PA). The NeuroStar TMS Therapy system is a noninvasive therapeutic device for the treatment of depression. It operates by applying a focused, pulsed magnetic field that inductively stimulates cortical neurons associated with depression.
NeuroStar TMS Therapy system, manufactured and submitted by Neuronetics Inc. (Malvern, PA). The NeuroStar TMS Therapy system is a noninvasive therapeutic device for the treatment of depression. It operates by applying a focused, pulsed magnetic field that inductively stimulates cortical neurons associated with depression.
Supply and design credit to Juliana Design Group Inc. (Exton, PA), Sparton Medical Group (Strongsville, OH), Molex Corp. (Napersville, IL), Gharieni GmbH (Moers, Germany), HS Design Inc. (Gladstone, NJ), Enser Corp. (Cinnaminson, NJ), Edge Medica (Newtown, PA), and Syncro Technology Corp. (Langhorne, PA).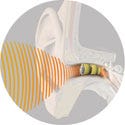 Lyric hearing aid, manufactured and submitted by InSound Medical Inc. (Newark, CA). The Lyric hearing aid is an invisible, extended-wear hearing device. Its unique design and deep canal placement provides natural sound quality for up to 120 days at a time.
Lyric hearing aid, manufactured and submitted by InSound Medical Inc. (Newark, CA). The Lyric hearing aid is an invisible, extended-wear hearing device. Its unique design and deep canal placement provides natural sound quality for up to 120 days at a time.
Supply and design credit to Center for Medical Device Innovations Inc. (Dublin, CA). NuStep T5XR recumbent cross trainer, manufactured and submitted by NuStep Inc. (Ann Arbor, MI). The NuStep T5XR recumbent cross trainer is a rehabilitation-exercise machine that combines a comfortable, safe position with total body motion for rehabilitation and fitness at healthcare facilities, at home, and in wellness and fitness centers.
NuStep T5XR recumbent cross trainer, manufactured and submitted by NuStep Inc. (Ann Arbor, MI). The NuStep T5XR recumbent cross trainer is a rehabilitation-exercise machine that combines a comfortable, safe position with total body motion for rehabilitation and fitness at healthcare facilities, at home, and in wellness and fitness centers.
Supply and design credit to Sundberg-Ferar Inc. (Walled Lake, MI), DeForest Engineering (Ann Arbor, MI), Griswold Engineering Inc. (Plymouth, MI), Humantech Inc. (Ann Arbor, MI), Keystone Product Development (Kalamazoo, MI), SMT Engineering LLC (Appleton, WI), Ottum Research & Consulting (Saline, MI), and Vaupell Midwest Molding & Tooling (Constantine, MI). Provent professional sleep apnea therapy, manufactured by Ventus Medical Inc. (Belmont, CA). Entry submitted by LUNAR (San Francisco). Provent is a prescription treatment for obstructive sleep apnea. Attached over the nose using a hypoallergenic adhesive, the device uses a novel valve mechanism to create expiratory positive airway pressure, which helps to keep the airway open.
Provent professional sleep apnea therapy, manufactured by Ventus Medical Inc. (Belmont, CA). Entry submitted by LUNAR (San Francisco). Provent is a prescription treatment for obstructive sleep apnea. Attached over the nose using a hypoallergenic adhesive, the device uses a novel valve mechanism to create expiratory positive airway pressure, which helps to keep the airway open.
Supply and design credit to LUNAR (San Francisco). Whiz Freedom hygienic urine director, manufactured and submitted by Jbol Ltd. (Oxford, UK). The Freedom hygienic urine director is a hydrophobic, antibacterial, and ecofriendly device that enables women to urinate standing or sitting, indoors or outdoors, without undressing. It is suitable for use by incontinent or mobility-impaired users.
Whiz Freedom hygienic urine director, manufactured and submitted by Jbol Ltd. (Oxford, UK). The Freedom hygienic urine director is a hydrophobic, antibacterial, and ecofriendly device that enables women to urinate standing or sitting, indoors or outdoors, without undressing. It is suitable for use by incontinent or mobility-impaired users.  ClearCut Oval Burrs, ClearCut Round Burrs, and ClearCut SLAP Burrs, manufactured and submitted by Arthrex Inc. (Naples, FL). ClearCut Burrs are a line of single-use, rotary instruments that provide flush cutting and dual-suction pathways for precise resection of target bone, while maintaining visibility during arthroscopic shoulder and knee surgery.
ClearCut Oval Burrs, ClearCut Round Burrs, and ClearCut SLAP Burrs, manufactured and submitted by Arthrex Inc. (Naples, FL). ClearCut Burrs are a line of single-use, rotary instruments that provide flush cutting and dual-suction pathways for precise resection of target bone, while maintaining visibility during arthroscopic shoulder and knee surgery.
Supply and design credit to B&M Precision (Ruskin, FL), Somerset Plastics (Middletown, CT), and SABIC Innovative Plastics (Pittsfield, MA). Mic and Mic-Key introducer kit, manufactured by Kimberly-Clark Health Care (Roswell, GA). Entry submitted by Three Atlanta (Atlanta). The Mic and Mic-Key introducer kit enables physicians to safely and efficiently place Mic and Mic-Key balloon-retained enteral feeding tubes, which are used to deliver nutrition and medication to patients requiring long-term nutritional support.
Mic and Mic-Key introducer kit, manufactured by Kimberly-Clark Health Care (Roswell, GA). Entry submitted by Three Atlanta (Atlanta). The Mic and Mic-Key introducer kit enables physicians to safely and efficiently place Mic and Mic-Key balloon-retained enteral feeding tubes, which are used to deliver nutrition and medication to patients requiring long-term nutritional support.  PolarWand endoscopic cryotherapy system, manufactured by G. I. Supply (Camp Hill, PA). Entry submitted by Bolt (Charlotte, NC). The PolarWand endoscopic cryotherapy system offers a simple and effective treatment modality for diffuse mucosal lesions of the GI tract.
PolarWand endoscopic cryotherapy system, manufactured by G. I. Supply (Camp Hill, PA). Entry submitted by Bolt (Charlotte, NC). The PolarWand endoscopic cryotherapy system offers a simple and effective treatment modality for diffuse mucosal lesions of the GI tract.
Supply and design credit to Bolt (Charlotte, NC) and Nexcore Technologies Inc. (Waldwick, NJ). Pulsar generator and PlasmaBlade disposable surgical cutting and coagulation devices, manufactured by Peak Surgical Inc. (Palo Alto, CA). Entry submitted by California MedTech LLC (San Diego). The surgery system includes the Pulsar generator, which generates pulsed plasma energy, and the PlasmaBlade family of disposable cutting tools. It is designed to provide the control of a scalpel and the bleeding control of traditional electrosurgery.
Pulsar generator and PlasmaBlade disposable surgical cutting and coagulation devices, manufactured by Peak Surgical Inc. (Palo Alto, CA). Entry submitted by California MedTech LLC (San Diego). The surgery system includes the Pulsar generator, which generates pulsed plasma energy, and the PlasmaBlade family of disposable cutting tools. It is designed to provide the control of a scalpel and the bleeding control of traditional electrosurgery.
Supply and design credit to California MedTech LLC (San Diego).
You May Also Like
.png?width=300&auto=webp&quality=80&disable=upscale)
