Technology Forecast: New Prospects for Medical Devices in 2000 : MAKING NANOSTRUCTURES THAT ASSEMBLE THEMSELVES : CREATING NEW THIN-FILM MATERIALS
Medical Device & Diagnostic Industry MagazineMDDI Article IndexOriginally Published January 2000TECHNOLOGY 2000Medical devices in the next century will take advantage of stronger, lighter materials, nanoscale components, and faster processing capabilities.
January 1, 2000
Medical Device & Diagnostic Industry Magazine
MDDI Article Index
Originally Published January 2000
Medical devices in the next century will take advantage of stronger, lighter materials, nanoscale components, and faster processing capabilities.
Predictions of what medical care may be like in coming decades offer dramatic images of advanced healthcare. Among the visions of things to come are hospital ventilation systems capable of monitoring ambient air to identify the presence of visitors who may be capable of spreading infectious disease within the facility, implanted insulin reservoirs or active nanocapsules of pancreatic cells to treat diabetic patients, and implantable devices capable of restoring hearing or vision. Other systems would allow physicians miles removed from their patients to both monitor their condition and provide therapy. On a more fanciful note, Jurgen Bey, a member of the Dutch collective Droog Design, has suggested that, in the future, human bodies might be used as time capsules by taking advantage of prostheses and implants. Described in a recent New York Times Magazine article, Bey's concept entails engraving information that could conceivably prove valuable upon exhumation to future populations on implantable devices such as pace-makers, joints, and dental implants.
Although it may be questionable whether future generations will have much interest in details inscribed on implanted medical devices, the stories that encompass the passing of conventional medical devices into a new age of microelectronics, artificial intelligence, and advanced materials are worth exploring. There will undoubtedly be significant changes in most categories of medical devices. This article, however, will focus on five areas: orthopedics, gene therapy and tissue engineering, functional electrical stimulation, sensors, and endoscopy.
ORTHOPEDICS AND HUMAN PERFORMANCE ENGINEERING
Prosthetics and orthotics engineering has undergone tremendous technical growth in the last few decades. Significant advances in materials technology, microprocessors, and design systems are resulting in devices that are stronger, lighter, and function in a more natural manner.
Increasing use of computer-based design systems has had a profound influence on the work of prosthetists as well as orthotists—yielding a unique blend of traditional methods and advanced technology. Alan Turner-Smith, of the King's Healthcare Rehabilitation Centre (London) has been involved with the center's Telemate program—European-wide network that has been established to share multidisciplinary training and education in assistive technology. Asked to identify the areas where the most dramatic advances are being seen, he replies: "Service delivery is the area being advanced. That is, device fitting and efficiencies of not having to keep plaster casts, and being able to rectify existing designs easily."
He suggests that the current technologies are a blend of the traditional craft of the prosthetist and orthotist with the application of high-technology solutions. He predicts that "The balance of craft and technology will continue, but software improvements—speed, quality of rendition of 3-D objects, possibilities of virtual reality, and force feedback—will make the interface more intuitive for the prosthetist."
Turner-Smith also suggests that the greatest challenge to prosthetics development in the 21st century will be personalized design: "That is, use of composite materials and control systems to solve individual demands at the cost of mass-produced devices. Prosthetists will have to either get it right the first time or make the solution so cheap that the represcription is not costly."
Human performance engineering encompasses a broad range of biomedical engineering interests. Its focus is on enhancing the performance and safety of humans executing tasks. George V. Kondraske, director of the Human Performance Institute at the University of Texas (Arlington, TX) suggests that the instruments used to measure human structure and performance have been improving rapidly in recent years—equaling advances in base technologies such as sensors, signal conditioners, and microprocessors. He describes the 1990s as a decade of change in which off-the-shelf components began to replace fabricated devices and systems—a trend that is likely to spread.
Despite recent technological advances, however, some experts have noted that an imbalance exists with regard to the commercial availability of needed tools for the field. According to Kondraske, the situation will improve in the future but "commercial availability will still be 'behind.'" He explains that "The reasons are complex and, in my opinion, mostly related to lack of overall conceptual frameworks that are employed on a widespread basis. There is activity ... that I believe is drawing attention to such issues and moving things in the right direction. However, the majority of researchers are content to pursue avenues of research that ignore such issues.... On the other end of the spectrum are researchers and clinicians who would be the potential users of such commercially available technology, but have not had a thorough exposure to the issues at play and therefore don't know how to specify exactly what they want. This keeps things confusing for those likely to invest in the commercialization." Kondraske believes that "the majority of the issues are now identified and reasonable answers are at hand, but that widespread dissemination of all this has not yet happened. Furthermore, the dissemination process is complicated by the fact that there are so many different disciplines that all deal with human performance, including neurology, orthopedics, sports medicine, and rehabilitation engineering."
Kondraske further notes that the field is still relatively young in nature and is following a natural maturing process, but is "not at all yet mature." He explains: "One example is that nearly every field dealing with human performance has publications within it that deal with instrumented measurement of 'something' related to human performance. A number of these discussions are actually in textbooks and not merely in journal articles. A second example is that there appears to be an increasing awareness regarding the need to deal with issues such as standardization. If a field is not in a maturing process, such issues don't even get recognized. A third example is that some major organizations have embarked on projects related directly to human performance measurement. They seem to recognize that progress has occurred and it is time to 'take a new look.' For example, about two years ago, the Social Security Administration started a project of reengineering the disability determination process. Again, due to limited widespread dissemination and consideration of many of the conceptual issues, I don't see the outcome of any of the current efforts leading to any panacea. However, these are signs of the maturing process."
Kondraske views the greatest challenge to human performance engineering in the next century to be dealing "with the complexity of the human system and its performance in an organized, systematic way—and for a general consensus to be reached regarding what that way would be. In my opinion, this will require a sophisticated view of the human that is rather different than that which is traditionally put forth in the medical world. We will get something that we don't have now, such as the ability to quantitatively characterize performance of different subsystems in an efficient, viable way, and to use such characterization for a wide range of 'long dreamed of' predictive uses." These might include predicting driving ability, sports performance, and the ability of an impaired person to live independently, he adds.
He suggests that the field's greatest potential is "wherever there is a human and a task." He adds that "This is everywhere. Without specifying all the reasons why, I believe that the greatest short-term potential is in medically related applications, where measurements and special analysis software based on new concepts can be combined to achieve functionality beyond that of simple measurements—that is, the assessment of the measurements. A close second is the sports field where acceptance of new methods may be easier to achieve."
As tomorrow's medical devices and related components become smaller in size, greater emphasis will be placed on microengineering and microsystems technology. Current efforts in these fileds are focused in part on lithography techniques that can be applied to microfabrication challenges. A recent development by researchers at Princeton University (Princeton, NJ) involves a technique that provides the basis for self-assembly of polymer microstructures. Their success in creating ultrasmall plastic structures involves a novel technique that they believe will prove less expensive and more versatile than previous methods. The nanofabrication technique is expected to aid development of a new generation of miniature device applications, ranging from microprocessors to devices for sorting DNA molecules. Led by Stephen Chou, PhD, professor of electrical engineering at Princeton, the group found that, working with little specialized equipment, a flat sheet of plastic resin could be coaxed to assemble itself into a minute, perfectly ordered array of pillars that are approximately one-half micron in height and width. Chou believes that the technique can be refined to yield even smaller structures. The researchers accidentally discovered the technique while working on another nanofabrication process called imprinting, which was also invented by Chou. The researchers were pressing a mask into polymer when dust prevented the two pieces from coming together. When they later examined the polymer, it contained a pattern of pillars--even though the mask had never touched it. In addition to growing by themselves, the pillars had arranged themselves into a perfectly ordered array. "It was a very surprising discovery. No one had ever seen such a thing," says Chou. Chou dubbed the new approach LISA (lithographically induced self assembly). Although the initial research involved use of a polymethylmethacrylate, the LISA process is expected be applicable to other polymers and, perhaps, single-phase materials such as semiconductors, metals, and biological materials, says Chou. He suggests that, "with proper design, a single crystal lattice of a pillar array with predetermined diameter, period, location, and orientation could be achieved over an entire wafer." Chou believes that the pillar pattern would be appropriate for a number of applications. One long-term application is in the design of ultrasmall circuits, according to the researchers. "Using the LISA process, you can fabricate your wires first, then it will assemble your devices between the wires on its own," says Chou. He also suggests that LISA could be much better suited to mass production than the most common nanofabrication technique, photolithography. So far, LISA cannot make features as small as those produced by photolithography, but that may change, Chou believes. One hope is to set up a repeating process where a relatively large mask makes many pillars, which would then be used as masks to make a new set of even smaller pillars. "In principle, you can get smaller and smaller and smaller things," he says. Currently, Chou is testing LISA with the same material used in Plexiglas, but he believes the technique will work with metals and other nonpolymer materials. |
GENE THERAPY AND TISSUE ENGINEERING
Much of the emphasis in current medical research is not on finding cures for disease, but on developing new methods for predicting and preventing disease. This is particularly true in the current efforts involving gene therapy. Programs such as the Human Genome Project have been focused, at least in part, on increasing understanding of our genomic structure so that disease processes can be more clearly understood and patients at risk for a given disease can be more easily identified.
In addition, tissue engineering is said to be among the most rapidly growing fields within biomedical engineering. It is expected to continue to play a significant role in cell and gene therapies over the next few years. Tissue engineering, in essence, involves the application of certain principles of biology and engineering to the development of substitutes capable of restoring, maintaining, or improving tissue function. There are two widely recognized categories of tissue engineering: in vitro construction of bioartificial tissues, and in vivo alteration of cell growth and function. Both these areas of interest are undergoing growth prompted by advances in various areas of technology, ranging from nanotechnology and computers to new materials.
According to François Berthiaume, PhD, of the Harvard Medical School and Shriners Burns Hospital (Boston), "The construction of tissues in vitro remains a daunting challenge because of the difficulty in overcoming transport limitations through tissue cell mass. There are a few cases where this is not a problem, such as in cultured skin, which is produced as thin layers of only a few cells. David T. Mooney, of the Massachusetts Institute of Technology (Cambridge, MA) adds, "A significant challenge in fabricating devices is either to develop processing techniques for natural biomaterials that allow reproducible fabrication on a large-scale basis, or to develop materials that combine the advantages of synthetic materials with the biologic activity of natural biomaterials." He further suggests that, in order to accomplish this goal, CAD/CAM techniques may be employed successfully in the future.
Berthiaume suggests that micropatterning may also prove to be a valuable method of improving tissue construction. "One of the most important recent advances in in vitro tissue construction is the development of techniques to micropattern cells. These techniques, adapted from those used in the semiconductor industry to generate computer chips, allow one to deposit cells on a surface at precise locations," says Berthiaume. "These methods are currently limited to two-dimensional surfaces, although there are efforts to develop such approaches in three dimensions."
He explains that micropatterning technologies offer the potential to improve tissue engineering in a number of ways. "First, by controlling the location of cells, it is possible to create microchannels reminiscent of capillaries in vivo, which could improve nutrient transport. Second, micropatterning can be used to maximize cell-cell interactions between two different cell types. For example, hepatocytes cultured on plastic exhibit much better function in the presence of a feeder layer of mesenchymal cells. Direct contact between the mesenchymal cells and hepatocytes is required for this effect to take place. Third, in applications where cell migration plays an important role, such as nerve regeneration, patterned surfaces may help direct or speed up the migration process."
Berthiaume also identifies the use of stem cells as an emerging area of tissue engineering. Derived from the bone marrow or certain embryonic progenitor cells, they have the ability to differentiate into several types of cells. Because of this, says Berthiaume, "they could be used to create more-complex tissues than what is possible with current approaches using one or two cell types. Such cells could be seeded into polymer matrices where they could be induced to grow and differentiate in vitro prior to implantation. Alternatively, the polymer implants could be designed to recruit stem cells in vivo by releasing factors that specifically attract these stem cells. Ultimately, the new tissue will be made up of cells derived from the host, which avoids problems related to immune rejection. Further studies to elucidate the nature of these factors will be necessary to make such approaches possible."
Mooney suggests that tissue engineering could one day offer alternatives to whole-organ or tissue transplantation. Research in the area has been motivated to a great extent by the ongoing shortage of tissues available for transplantation, which has resulted in patient deaths from the lack of available tissue or use of suboptimal therapies because of the shortages.
Some research efforts have been focused on investigation of selective cell transplantation as an alternative to whole organ transplantation. There are a number of advantages to this approach. Using cell transplantation to reconstruct functional tissue in vitro would alleviate problems associated with donor organ shortages because the procedure would require only a small number of cells from the donor. In vitro expansion of the small amount of harvested cells would create potentially unlimited supplies of tissue. In addition, the risks typically associated with surgical procedures could also be decreased. The need for immunosuppression during transplantation of autologous cell transplants could also be reduced, according to most experts. Some suggest that, eventually, it will be possible for cells that have been harvested from a patient to be modified in order to replace defective genes prior to reimplantation.
Some investigators have focused research efforts on development of specialized vehicles for delivery of engineered tissue for cell transplantation. Researchers at Massachusetts General Hospital (Boston), for example, have used synthetic biodegradable polymer scaffolds as delivery vehicles for cell tissue. The technique allows cells to be delivered and immobilized at a specific site, and to act in the manner of a template for tissue development. The scaffold also provides a space in which the transplanted cells can reorganize into higher structures. The technique avoids long-term foreign body response by the patient's system because the scaffold eventually resorbs. The researchers have formulated a number of clinical applications that are undergoing investigation as a means of achieving permanent replacement of lost organ function.
The development of a number of medical devices has been made possible in part by the application of advanced polymer thin-film materials. Efforts to create new polymer thin-films by using blends of polymers, however, have been hampered by the challenge of blending certain polymers that simply do not mix. Physicists at North Carolina State University (NC State; Raleigh, NC) are collaborating with materials scientists at the State University of New York (SUNY; Stony Brook, NY) to address the problem. The team is focusing on the specific processes that are involved as the dimensions of polymer blends shrink. They are also exploring how enhanced thin-film materials can be generated by exploiting these processes. Says Harold Ade, MD, associate professor of physics at NC State, "We know that, as a material shrinks, its large-chain molecules—its polymers—no longer have room to 'stretch out' as they ordinarily would. This affects their spatial relationship to other polymers and, in some cases, the 'mixability' of the polymers themselves." Ade explains that the challenge to the researchers is to learn how to understand and control these effects and use them advantageously. The goal is to promote a consistent mixture of polymers throughout a thin-film blend without limiting the types of polymers used. The group is attempting to exploit the process of reduction in entropy, described as a measure of the number of possible molecular arrangements in a material. They indicate that this process occurs as a result of miniaturization. The researchers recently reported that, for the first time, highly dissimilar polymers can be completely blended into a thin film through the application of entropy-reduction principles. Ade explains that "It's sort of like getting water and oil to mix. The beauty of nature is that, if a polymer blend is shrunk small enough, the emulsifier utilized is essentially prevented from associating with other emulsifier molecules. There's no room for the emulsifier to arrange itself in such a way due to the confined space." When emulsifiers are used to mediate polymer blending and stabilize mixtures, the emulsifier molecules can associate with other emulsifiers. When this occurs, they lose much of their stabilizing ability and the polymers can separate. The result is most often unacceptably large modulations on the surface of the material and inconsistent internal structure. Flaws of this type usually render a material useless for most applications that require a perfectly flat surface and where tight structural tolerances must be satisfied. Describing the method of polymer blending, Ade says "The thin-film polymer blend we created was made from very dissimilar polymers but had a perfectly flat surface and a completely mixed, uniform structure when reduced to nanoscale. This is the first time we've seen that in highly immiscible systems." The scientists speculate that the technique should be applicable to most technological processes that rely on ultrathin polymer coatings. |
NEW TREATMENT OPTIONS IN CARDIOLOGY
Innovations in imaging techniques, minimally invasive surgery, and other areas of disease diagnosis and treatment are all shaping the future of cardiology. Among the most dramatic areas of current research, however, is the use of stents to open blood vessels and arteries that have been closed by a buildup of plaque or to strengthen tissue threatened by the presence of an aneurysm.
Medtronic Inc. (Minneapolis) recently received marketing clearance for its AneuRx stent graft system used to treat abdominal aortic aneurysms (AAAs), a bulge in the wall of an artery. Medtronic describes the technology as "the first new treatment option for AAA in 40 years." The formation of AAAs is generally associated with cellular changes caused by arteriosclerosis, which damages and weakens the artery wall. Timely diagnosis and treatment of the aneurysm are critical to preventing rupture of the aorta, which most often results in the patient's death.
 Medtronic's AneuRx system uses self-expanding diamond-shaped rings to create a "friction fit".
Medtronic's AneuRx system uses self-expanding diamond-shaped rings to create a "friction fit".
The company indicates that results from a prospective, nonrandomized, multicenter trial in the United States demonstrated the AneuRx stent graft to be as effective as conventional treatment of AAA. The firm claims use of the stent has the potential to cut major complications associated with cardiac surgery by half. Results of the study, which involved 482 patients, also suggest that the technique improved patient quality of life by reducing hospital stays from 9.3 days to 3.4 days and reducing the time to ambulation from 3.6 days to 1.4 days.
Says Christopher Zarins, MD, chief of vascular surgery at Stanford University School of Medicine (Palo Alto, CA) and clinical investigator in the study, "Endovascular repair of AAA is truly a breakthrough in that it offers significant potential benefits to patients. In addition to being as effective as open surgery, patients who received the AneuRx device also had fewer serious complications, spent less time in the hospital and ICU, and experienced faster recoveries."
Conventional treatment of aneurysms has entailed open surgical repair, with an average hospital stay of 7 to 12 days and a recuperative period that can last as long as six months. The procedure itself involves making a large abdominal incision and clamping the aorta above and below the aneurysm. Opening the aorta, a surgical graft is sewn in at the diseased site and the aorta is closed.
During the AneuRx procedure, an incision is made in each groin area. A delivery catheter is used to deliver the stent graft into the femoral artery and guide it through the aorta to the location of the aneurysm. The stent graft is placed within the aneurysm where it expands to fit within the diameter of the aorta. The result is a new path for blood flow and a significant reduction in the pressure on the aneurysm. The catheter is withdrawn after placement of the stent graft.
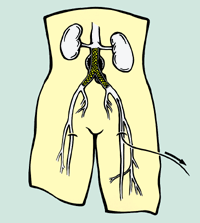 The AneuRx endovascular stent-graft system consists of catheter-mounted grafts that form new passageways to carry blood to the legs past potentially lethal aneurysms.
The AneuRx endovascular stent-graft system consists of catheter-mounted grafts that form new passageways to carry blood to the legs past potentially lethal aneurysms.
The AneuRx system uses self-expanding, diamond-shaped, nickel titanium stent rings to create a "friction fit" to anchor itself to the vessel wall without the need to puncture the vessel with hooks or barbs. Physicians can use extender cuffs to modify the length or diameter of the implanted graft as a means to address implant challenges and changes in aneurysm size and shape. The stent graft exterior is designed to prevent kinks or twisting, and device migration over time, which can require surgical repair.
FUNCTIONAL ELECTRICAL STIMULATION
Electrical stimulation has been found to be an effective means for restoring muscular function. Implantable functional electrical stimulation (FES) systems have been developed to restore and maintain control of bowel and bladder activity, lung function, and limb movement. All such systems operate by applying an electrical current to nerves through the use of implanted electrodes. Increased understanding of precisely how the human nervous system functions, coupled with technological improvements is providing the foundation for notable improvements in years to come.
Functional electrical stimulation (FES) technology has been successfully used in treating a number of medical conditions. FES has been a critical part of orthotic and prosthetic devices that can be used to restore ambulation and grasping functions to patients with spinal cord injuries. In addition, FES technology has been proven effective in treating epilepsy and tremors associated with Parkinson's disease. Other applications include control of incontinence and deep brain stimulation to relieve rigidity.
Looking to the future of FES applications, P. Hunter Peckham, director of the Cleveland FES Center, has suggested that advances in implantable stimulation methods are expected to occur in two areas: the integration of control and stimulation functions within implantable systems, and the potential applications of microinjectable neurostimulators.
A number of systems are currently in development in which external neuromuscular stimulators are capable of communicating with and controlling implanted sensors. A configuration of this sort would allow the stimulation device to both power the sensor and telemeter the data generated by the sensor to the controller located outside of the body. The controller would then be able to process the data and transmit appropriate functional commands back to the stimulation component. Such a device is under development at Case Western Reserve University (Cleveland) for more-advanced neuromuscular applications. The command data could be derived from various physiologic sources, including joint position or feedback control loops, according to Peckham.
Research at several centers is focusing on development of microinjectable neurostimulators--small capsules capable of being inserted through a tiny injector directly into muscle tissue for nerve activation. Numerous such devices could be placed in particular muscles and controlled via a single external coil that surrounds the implantation site.
About the Author(s)
You May Also Like

.png?width=300&auto=webp&quality=80&disable=upscale)
