PwC Analyst Considers Medtech Funding Climate
Q: Can you help me put the current VC funding climate in perspective? How does funding climate for medical device companies compare, from your perspective, with where we were five years ago? Ten years ago? Why is it different now?
November 10, 2011
MD+DI recently had the opportunity to interview Tracy Lefteroff, global managing partner of PricewaterhouseCoopers (PwC; San Jose) life sciences industry services, where he advises publicly held, privately owned, and venture-capital-backed life science companies. Lefteroff also serves on the board of directors at the Stanford Venture Laboratory and the California Healthcare Institute.
Q: Can you help me put the current venture capital (VC) funding climate in perspective? How does the funding climate for medical device companies compare, from your perspective, with where we were five years ago? Ten years ago? Why is it different now?
|
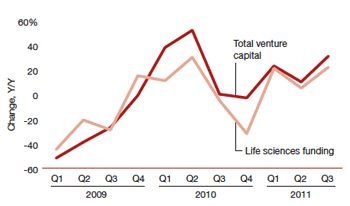
Life sciences funding compared with total venture funding from 2009 to the third quarter of 2011. Credit: Money Tree: Reaching for Growth report from PwC. |
A: What’s going on, number one, is 10 years ago, we were still seeing some IPO activity, albeit... after the Internet bubble. But at least in the life sciences base, we were still seeing some activity, and [the frequency of IPOs has] tailed off over the last 10 years. One factor that has really had a negative impact on funding for these young medical device and diagnostic companies has been the regulatory pathway through the FDA, which has been a negative factor in companies getting funded as well as to continue to fund existing companies. It’s been a real challenge—both on the device side and the biotech side. We are seeing the lack of transparency on the regulatory pathway through the FDA impacting these companies’ ability to raise money.
Q: Thomas Fogarty, whom I recently spoke with, largely blames the FDA for the conservatism in VC funding. How big of a role do you think regulatory concerns play in that?
A: It absolutely plays a big role. They’ve tried to do drastic change on the way that they allow medical devices to be cleared under 510(k)s. They are much more conservative on which device companies need a PMA. On the biotech side, the clinical trials have been an issue of contention at companies that believe that they have an agreement on endpoints, and then after they do the clinical trial, the FDA comes back and says, well, we want additional trials and additional endpoints that weren’t discussed up front when they first figured out their pathway. And all of those things cost the companies substantial amounts of money. Venture capitalists, if they have to put too much money into a deal, that obviously erodes the returns that they get, and decreases their incentive to invest in some of these companies, because they can’t get venture-type returns.
Q: Many big medical device companies now have their own venture arms. What do you think the import of that will be on the funding climate?
A: It should help with the additional money they are funding. The question is, are the large companies’ venture arms truly going to do very early stage and seed-stage type investing? Typically, what we have seen out of those companies, they also want to do later-stage investing, so that they can get a seat at the table of the companies that may potentially be acquisition targets for them. [This is] much closer to... those large companies’ goals, which is to bring additional products into their product line.
Q: What are we likely to see happen with VC funding moving into 2012?
A: Your crystal ball is as good as mine, but I would expect that if the FDA doesn’t get things turned around, we will probably see these [downward] trends continue, which means it’s going to be tougher to get funded if you are an early stage company.
Q: I’ve heard some anecdotal evidence that the FDA is acknowledging the importance of becoming more collaborative with the industry. What are the prospects that the tough environment out there will convince the FDA to address these issues?
A: We’ve seen these cycles before. The FDA has its highs and lows. The problem is that trying to change policy at the FDA is like trying to turn the Titanic, and so it just takes time for that to happen.
Q: What is behind the fall in funding in medical/health products and medical diagnostics in the third quarter of 2011?
A: You've got to remember that the third quarter was an extremely volatile time for the stock markets. So you didn’t see the number of IPOs that I think this sector would have liked to have seen. And, again, anytime you have volatility in the markets, it adds another layer of caution on top of the FDA problems and everything else. It’s one more reason for people to say, 'Let’s be patient and wait a little while before we deploy more capital.'
Q: Besides the VC funding changes, what other shifts do you think are important to the medtech sector?
A: Again, you have to get public markets back to where they are healthy again, without so much volatility and negativism about smaller-cap companies, which a lot of these device companies are. And you need to see, globally, business pick up for a lot of these companies so that sales continue to increase, profits increase, which will then attract later stage M&A activity, which all trickles down from there.
About the Author(s)
You May Also Like