Cutting-Edge Technology Enables the Deaf to Hear
July 1, 1999

Cutting-Edge Technology Enables the Deaf to Hear
Karim Marouf, Managing Editor
Many medical OEMs push the boundaries of technology to develop devices that restore functions or abilities. The suppliers that partner with them are challenged to pioneer their own technologies in order to provide solutions for the device manufacturers' needs. But the rewards for both companies are many.
Not only do suppliers and manufacturers get the satisfaction of collaborating to make a product that improves people's lives, but they can also apply the new technology to other devices in medical and other industries. Such a mutually beneficial relationship occurred during the development of the Clarion cochlear implant, built by Advanced Bionics Corp. of Sylmar, CA, with the help of its supplier, AVX Corp. of Myrtle Beach, SC.
Some deaf people benefit little, if at all, from hearing aids, and the Clarion cochlear implant was designed specifically to help them. Unlike a traditional hearing aid, the device actually bypasses much of the auditory system and delivers electrical stimulation directly to the hearing nerves of the inner ear.
The "Bionic Ear"
A remote speech processor, connected to a quarter-sized headpiece, converts incoming sound into electronic codes. The headpiece receives the sound information from the processor and transmits it via radio waves to a cochlear implant. The implant is a small microcircuit that is placed under the skin and attached to the skull.
An electrode array of very thin wires extends from this implant to the inner ear through a hole drilled into the skull. The array inserts directly into the inner ear and provides the interface between the device's electronics and the human nervous system. Electrical current excites the inner ear's nerve fibers, and these in turn deliver the signals to the brain, where they are interpreted as sound.
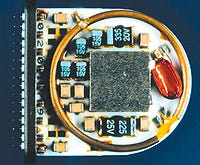 AVX Corp. developed the miniature capacitors for the Clarion system's headpiece.
AVX Corp. developed the miniature capacitors for the Clarion system's headpiece.
The technology achieves impressive results. "I'm totally deaf in both ears," says implant user Doug Lynch, manager of marketing communications for Advanced Bionics. "I'm representative of an individual that you can strap as many hearing aids on as you want without any benefit to my hearing. But with the implant I'm able to hear normally."
Although the principles of this technology have been around since the 1970s, the technology has progressed dramatically in just the past five years. Advances in electronics miniaturization were the primary catalyst for Advanced Bionics.
For help in achieving the necessary miniaturization, Advanced Bionics went to its supplier, AVX Corp., a manufacturer of a variety of passive and electromechanical components such as resistors, ultraminiature fuses, and connectors. AVX developed the miniaturized TAC tantalum capacitor to fit into the headpiece unit.
The temperature and voltage stability of tantalum enabled the designers to achieve miniaturization without compromising performance. But AVX had to develop new methods of making the capacitor. Craig Hunter of AVX explains: "The normal method of making a tantalum capacitor wouldn't have worked because the capacitor would've been too big. A completely new production method was developed. It involves a new termination—how it's connected to the PCB." The capacitor is 10 times more powerful than a traditional tantalum capacitor of its size. It measures only 0.06 x 0.03 in.
A Challenge for the Future
"I'm fascinated by the Clarion device, as much from the perspective of someone who uses it as well as from someone who's just interested in seeing the incredible things being done in biotechnology," says Lynch.
"From our side, it's great when you cannot just push the boundaries, but in the end can achieve something substantial," says Hunter. "You're not just making a smaller cell phone or something like that. You're enabling a deaf person to hear. That's incredibly rewarding, and something everyone at our company can be proud of."
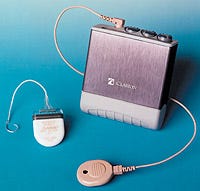 The Clarion system delivers electrical stimulation directly to the hearing nerves of the inner ear.
The Clarion system delivers electrical stimulation directly to the hearing nerves of the inner ear.
The Clarion cochlear implant was approved by FDA for children in 1997 and for adults in 1998, and since then thousands of people have had the device implanted. A possible future improvement may involve having the headpiece also implanted. This will only be possible with further miniaturization of digital processing chips and other components, and that will require a leap in technology.
Advanced Bionics won't shy away from taking on such challenges in the future. Its suppliers, including AVX, continue to offer support by overcoming the technological barriers needed to achieve success.
The rewards will be far-reaching. Hunter concludes: "Medical companies like Advanced Bionics create developments so revolutionary that they force us to work hard to improve our own technology and to really push the boundaries. In the end we doubly benefit since the next logical step after developing a solution to fit their needs is to see in what other industries our new technology can be applied."
Copyright ©1999 Medical Product Manufacturing News
You May Also Like


