Allergan Aesthetics Set to Acquire Soliton for $550M
The acquisition would give AbbVie’s Allergan Aesthetics access to Resonic, a non-invasive treatment for the short-term improvement in the appearance of cellulite.
May 10, 2021
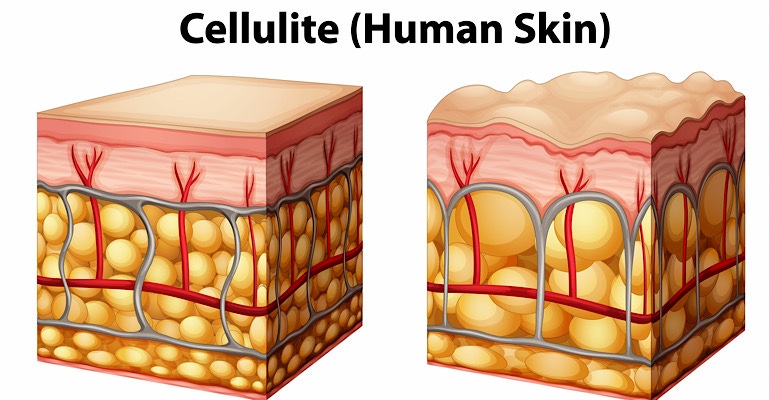
AbbVie’s Allergan Aesthetics is expanding its body contouring treatment portfolio with the proposed acquisition of Soliton for $550 million. The deal would Allergan Aesthetics access to Resonic a rapid acoustic pulse device, which recently received FDA clearance.
Resonic is a non-invasive treatment for the short-term improvement in the appearance of cellulite. The device disrupts targeted cellular structures and connective tissue, physically impacting the fibrous septae beneath the skin that contribute to the dimpled appearance of cellulite. In clinical trial data submitted to FDA, after a single treatment session Resonic demonstrated significant improvement and strong patient satisfaction with 92.9% of subjects agreeing or strongly agreeing their cellulite appeared improved.
"There is a huge unmet need to address cellulite and effective treatments have been elusive and frustrating for consumers," said Carrie Strom, President, Global Allergan Aesthetics and Senior Vice President, AbbVie. "Soliton's technology offers a new, completely non-invasive approach with clinically-proven results to reduce the appearance of cellulite with no patient downtime. The addition of this technology complements Allergan Aesthetics' portfolio of body contouring treatments. Healthcare providers will now have another option to address consumers' aesthetic concerns."
This is the second significant acquisition in the medical aesthetics company in the past few days. A little more than a week ago, Cynosure entered into an agreement to acquire the MyEllevate Surgical Suture System, a light-guided technology for use in soft tissue approximation and the elevation of sub-dermis and underlying muscle.
About the Author(s)
You May Also Like




