NeuroOne Tried Again – And Succeeded
FDA shot down the company's earlier bid for extended use of its Evo sEEG, but NeuroOne didn't give up.
November 1, 2022

Persistence has paid off for NeuroOne Medical Technologies, a company that develops electrode technology for diagnostic brain mapping procedures.
NeuroOne's Evo sEEG system had previously been FDA cleared for less than 24 hours use, but earlier this year FDA shot down the company's bid to extend its use. Instead of giving up, the company resubmitted its 510(k) application for less than 30-day use, along with additional biocompatibility testing, and the application was successful.
"I am extremely proud of the entire NeuroOne team and their relentless pursuit of this clearance," CEO Dave Rosa said. "Despite the challenges we faced, our team remained focused and persistent in driving this successful conclusion. This is clearly our most exciting and important accomplishment to date. We are now able to advance our commercialization efforts in partnership with Zimmer Biomet, our distribution and development partner."
The Evo sEEG system represents the Eden Prairie, MN-based company's second FFDA cleared product. NeuroOne's electrode technology is designed to address a $100 million worldwide market for patients requiring diagnostic brain mapping procedures. Unlike cortical electrodes, sEEG electrodes provide a similar function at the subsurface level of the brain by using a much less invasive process that does not require removal of the top portion of the patient's skull.
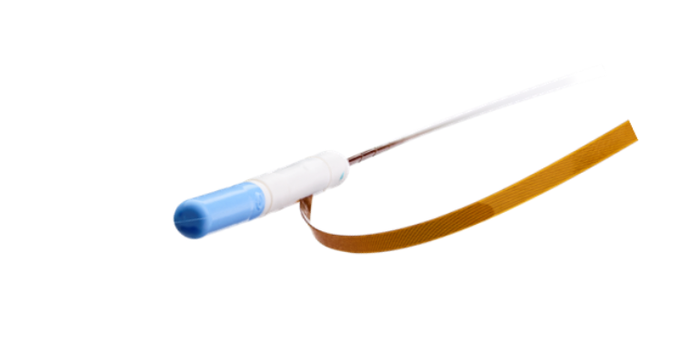
Above: an image of the NeuroOne sEEG electrode showing the stylet and ribbon end of the device close up.
The company's Evo Cortical and sEEG electrodes are a portfolio of hi-definition thin film electrodes. According to NeuroOne, potential advantages of these electrodes include increased signal clarity and reduced noise; better tactile feedback during insertion into brain tissue; and faster order fulfillment due to an automated manufacturing process.
NeuroOne is also developing a pipeline of therapeutic electrode technologies for brain tissue ablation and chronic stimulation use for deep brain stimulation and spinal cord stimulation for chronic back pain. These therapeutic electrode technologies represent addressable markets valued between $500 million and $6 billion.
About the Author(s)
You May Also Like


.png?width=300&auto=webp&quality=80&disable=upscale)

