Is Medtech's Next Big Thing Years Away?
December 3, 2015
It could take years before transcatheter mitral valve replacement truly hits the market, an analyst in the space is warning.
Nancy Crotti
|
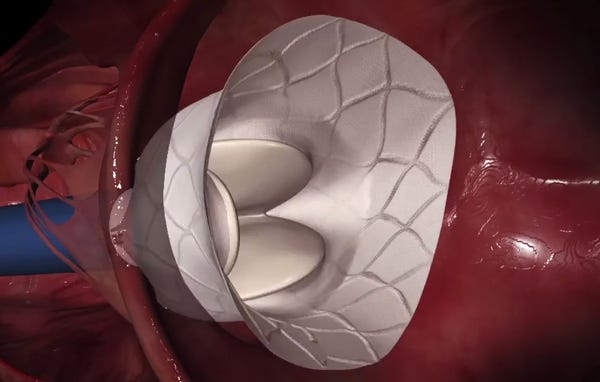
This screen grab from an animation shows the Tendyne Bioprosthetic Mitral Valve deploying. Abbott announced in July it would acquire the technology for $225 million. (Image courtesy of Tendyne Holdings) |
Device makers are sinking hundreds of millions of dollars into technology to fix leaky mitral valves, although they're years away from bringing the technology to market.
The brass ring they're chasing is a transcatheter mitral valve replacement device, designed to help patients whose mitral valve allows blood to leak back into the heart.
A minimally invasive device to treat mitral valve disease might give critically ill patients ineligible for open-heart surgery a chance at survival. However, bringing such a device to market "has been very technically challenging," said market analyst Jeff Jonas of Gabelli Funds (Rye, NY).
|
Jeff Jonas |
Mitral valve disease is the most prevalent valve disease in the world, according to Abbott, which announced in July that it would buy Roseville, MN-based Tendyne Holdings and its minimally invasive mitral valve replacement technology for $225 million. In a separate transaction, Abbott is spending an undisclosed sum to secure an option to buy Cephea Valve Technologies (San Jose, CA), which has been developing a catheter-based mitral valve replacement therapy.
Abbott already sells its MitraClip device, used to treat certain types of mitral valve disease, in the U.S. and many parts of the world. The company expects minimally invasive mitral valve repair and replacement to become a multi-billion dollar market over the next 10 years.
Medtronic Plc, Edwards Lifesciences and HeartWare International Inc. have also leapt into the mitral valve market, to mixed reviews. Dublin-based Medtronic plans to sink $548 million into Twelve, Inc. (Redwood, CA) and its transcatheter mitral valve replacement technology. Edwards plans to spend $400 million for CardiAQ Valve Technologies and its mitral valve replacement system. HeartWare has drawn investor ire for its proposal to acquire Israeli firm Valtech Cardio Inc. for $929 million.
Edwards is "probably the most advanced of the group," said Jonas, an analyst who follows Medtronic and Edwards. CardiAQ already has an FDA Investigational Device Exemption approval to conduct an early feasibility study of up to 20 patients, and plans to initiate a CE Mark study in Europe.
Even with these human trials on the horizon, FDA approval for a mitral valve device is probably about four years away, Jonas said. Companies in the space will need time to gather follow-up data from trial participants. Jonas suspects the technology would advance quickly after that, and prove very lucrative to manufacturers.
"I do think it's promising and worth billions of dollars when it's done," he said. "It's still not really on my radar screen as something to invest for or get overly excited about."
Nancy Crotti is a contributor to Qmed and MPMN.
Like what you're reading? Subscribe to our daily e-newsletter.
About the Author(s)
You May Also Like