Repositionable TAVR Device Wins CE Mark
January 9, 2014
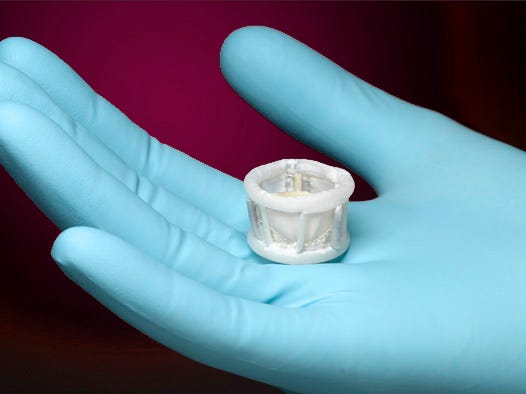
Direct Flow Medical (Santa Rosa, CA) has obtained the CE Mark for its 29-mm transcatheter aortic valve replacement device, which it hails as the first fully repositionable percutaneous heart valve. The ability to reposition the Direct Flow Medical system enables clinicians to fine tune its performance following a hemodynamic assessment and prevent valve leakage. In addition, the device can also be deflated and removed if necessary. The company believes the ability to prevent such leakage is a key advantage for the product, as valve regurgitation has been problematic for some patient and correlated with higher long-term mortality.
|
|
The device features a polyester cuff and bovine pericardial leaflets of a surgical heart valve, which it combines with the deployment attributes of non-compliant percutaneous coronary intervention technology. The device is inserted transfemorally using a flexible, 18 French delivery system. It has no metal parts.In the European market, the company is competing against the likes of Medtronic and Edwards Lifesciences. Founded in 2004, the company now has roughly 130 employees. Direct Flow Medical has also announced that it has finished enrollment in its SALUS domestic feasibility trial of TAVR device. A U.S. pivotal trial is planned for this year. At the 2013 Transcatheter Cardiovascular Therapeutics held in San Francisco, the company shared six-month data from its Discover CE Mark study, which indicated the technology's ability to nearly eliminate significant aortic valve regurgitation.
About the Author(s)
You May Also Like

.png?width=300&auto=webp&quality=80&disable=upscale)
