PMCF Surveys: Deciding upon the Correct Approach
Post-market clinical follow-up (PMCF) plans need to be made now to satisfy the EU MDR’s expectation for post-market clinical data.
August 9, 2021

The EU Medical Device Regulation (EU MDR) has now been live for more than two months since taking effect May 26. In order to continue to CE mark their devices, medical device manufacturers are being asked to be more proactive in assessing the safety and performance of their products, backing this up with clinical data and post-market monitoring. The data collected will be used to update risk management and the clinical evaluation of each device.
There are many ways to collect this post-market data, and it is key to understand which approach(es) will be most appropriate when creating the clinical strategy and corresponding post-market clinical follow-up (PMCF) plans. Some of the most common approaches are listed below:
Randomized clinical trials (RCTs)
Registries
Literature reviews
Surveys
PMCF via End-User Surveys
When weighing which approach(es) to take, manufacturers should consider the balance between the level of evidence required and the time and effort to collect this evidence. We at Purdie Pascoe took the time to survey some of our PMCF survey clients and found that PMCF surveys are a very appealing route to take, given that they offer a useful, cost-effective, and timely approach to high-quality PMCF data collection. RCTs and registries, on the other hand, can be time consuming and costly.
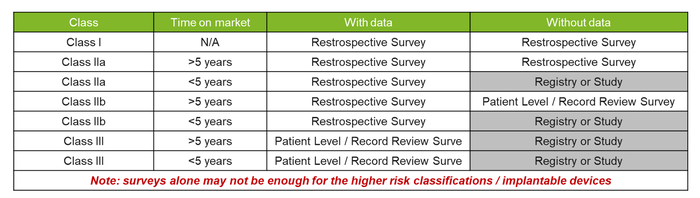
The justification for end-user surveys, and the type of survey, will depend on the type of medical device, including the risk classification and previous data obtained. They can be used alongside other PMCF methods to strengthen the evidence of data for all risk classes. However, using them as the sole PMCF data source would not be recommended.
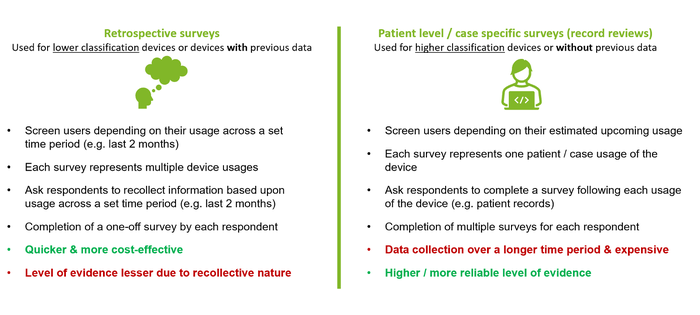
Retrospective vs. Patient Level / Record Review PMCF Surveys?
Not only should manufacturers consider whether PMCF surveys would be an appropriate source of PMCF data, but they should carefully select the type of survey. As Notified Bodies (NBs) begin to provide more and more guidance and set their expectations as to what is required, there has been a clear push to try and gather more specific/real-time data for higher-risk classification devices or those with limited previous data. See below for a quick explanation on the potential survey approaches:
Finally, Why It Is Important to Start Thinking about This Now…
As a manufacturer you will have to comply with the EU MDR, and if not, some of your much-needed medical devices may be forced to come off the European market. It is not only important to begin your PMCF data collection, with surveys offering an extremely valuable tool, but it is vital to ensure that the approach is appropriate and will provide you with acceptable data for your submissions.
So make sure that you have everything in place to be compliant with the EU MDR, today.
About the Author(s)
You May Also Like



.png?width=300&auto=webp&quality=80&disable=upscale)
