Olympus to Pay Whopping $646 Million over Endoscope Kickbacks
March 1, 2016
The settlement of criminal and civil charges is the largest Anti-Kickback Statute payment ever made in the United States by a medical device company. It comes about a month and a half after a U.S. Senate report blasted Olympus for failing to alert the U.S. public, even as hundreds became infected with potentially deadly superbugs related to a type of endoscope called a duodenoscope.
Chris Newmarker and Brian Buntz
Adverse Events Linked to the Olympus TJF-Q180V Duodenoscope
Report Year | Death | Injury | Malfunction | Other |
2012 | - |
| 1 | - |
2013 | - | 2 | - | 2 |
2014 | - | 21 | - | 1 |
2015 | 6 | 85 | 2 | 1 |
Data in the table above compiled by Steve Gill, an independent industry consultant, from the MAUDE database
Olympus Corp. of the Americas--already under criticism for its handling of deadly superbug outbreaks related to a type of endoscope called a duodenoscope--will now have to pay a total $646 million to settle criminal and civil complaints that it paid kickbacks and bribes to health providers in both the United States and Latin America, the U.S. Justice Department announced Tuesday.
The Justice Department is describing the settlement as the largest ever paid by a medical device company under the U.S. Anti-Kickback Statute. Federal prosecutors say Olympus Corp. of the Americas, a subsidiary of the Japanese multinational, paid kickbacks that reached into the hundreds of thousands of dollars apiece that then allowed it to realize $600 million in sales and gross profits of more than $230 million.
|
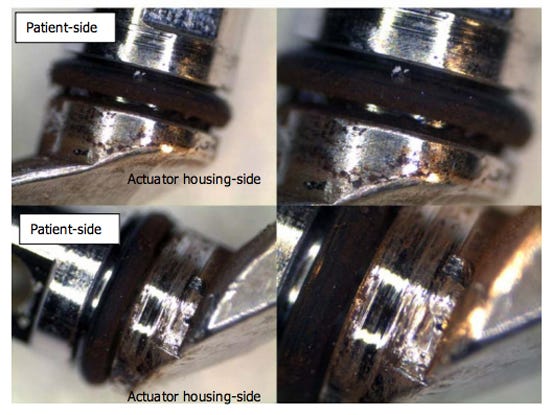
Pictures of a contaminated Olympus TJF-Q180V closed-channel duodenoscope, taken during a 2012 investigation in the Netherlands that Olympus participated in. The O-ring shows signs of wear, and the actuator-side area is heavily covered with brown scale. (Image from the Senate Health, Education, Labor, and Pensions Committee report) |
"Olympus Corp. of the Americas' and its subsidiaries' greed-fueled kickback scheme threatened the impartiality of medical decision-making and the financial integrity of Medicare and Medicaid," said agent Scott J. Lampert of the U.S. Department of Health and Human Services' Office of Inspector General.
Even worse, the Justice Department alleges that Olympus lacked training and compliance programs at the same time it was paying the bribes. Unlike other medtech companies, Olympus did not have a compliance officer until 2009, and did not hire an experienced compliance professional until August 2010.
"Unfortunately, Olympus is not unique in putting profits before people's lives," says Diana Zuckerman, PhD, president of the National Center for Health Research (Washington, D.C.) "But, we can hope that when companies get caught and fined, that has a chilling effect on other companies that are behaving similarly or considering doing so."
"The FDA's role in this is more upsetting, because the FDA was created to make sure that medical products are safe," Zuckerman adds. "The FDA is not doing a good job of making sure medical devices are safe, and they let Olympus off the hook instead of penalizing them. That exacerbates the problem, encouraging companies to get away with whatever they can. In contrast, a $646 million fine has a very substantial chilling effect."
Olympus in a statement shared by a spokesman acknowledged the company's responsibility for past conduct, saying that it "does not represent the values of Olympus or its employees." The company said that it is committed to complying with all laws and regulations and its own code of conduct, as well as achieving its mission to "help people around the world lead safer, healthier and more fulfilling lives."
The accusations related to the lack of training and compliance are especially serious in light of Olympus' problems with duodenoscopes, which are threaded down through the digestive tract and into the small intestine. The company failed to disclose the extent of contamination problems with a type of endoscope called a duodenoscope to FDA and the U.S. public, even as it issued "important safety advice" in Europe in 2013, and another European safety alert about tainted scopes in 2014, according to a report released in January by Sen. Patty Murray, D-WA, the top Democrat on the Senate Health, Education, Labor, and Pensions Committee.
It wasn't until early 2015 that enough news had been generated from deadly outbreaks to spark warnings from FDA, which divulged that Olympus' TJF-Q180V closed-channel duodenoscope had initially made it to market without a 510(k) clearance. By March, FDA was reporting that Olympus had "issued new, validated manual reprocessing instructions for the TJF-Q180V" as part of the review of a 510(k) application for the device, which FDA allowed Olympus to continue marketing while the application was under review.
Problems had been mounting for years with Olympus' TJF-Q180V duodenoscope, according to FDA MAUDE data compiled by Steven Gill, an independent industry consultant.
Virginia Mason Medical Center in Seattle and UCLA's Ronald Reagan Medical Center are among the hospitals that eventually reported Olympus scope-related deaths related to Carbapenem-resistant Enterobacteriacea (CRE). The U.S. Senate report estimates that 25 outbreaks sickening at least 250 patients in the United States and Europe between 2012 and 2015.
The accusations that the Justice Department outlines, meanwhile, paint a picture of a medical device company more focused on paying kickbacks to drum up sales. According to federal prosecutors, Olympus Corp. of the Americas' kickbacks included:
Giving a hospital a $5,000 grant to facilitate a $750,000 sale;
Holding up a $50,000 research grant until a second hospital signed an equipment purchase deal;
Paying to send three doctors to Japan in 2007 as a quid pro quo for their hospital's decision to switch from a competitor to Olympus;
Making a deal with a doctor with a major role in a New York medical center's buying decisions in which the doctor received free use of $400,000 in equipment for his private practice.
For those activities and more, Olympus Corp. of the Americas has agreed to pay a $312.4 million criminal penalty and an additional $310.8 million civil lawsuit settlement under the federal and various state False Claims Acts. The company must also adopt several compliance measures to remedy its problems.
On top of that, Olympus Corp. of the Americas' Olympus Latin America Inc. subsidiary will pay $22.8 million to resolve Foreign Corrupt Practices Act (FCPA) charges, according to the Justice Department.
"Olympus Latin America admitted to bribing publicly employed health care providers and hospital officials across Central and South America so that it could illegally win business and sell its products," said Principal Deputy Assistant Attorney General David Bitkower. "OLA's illegal tactics in Central and South America mirrored Olympus's conduct in the United States."
Learn more about cutting-edge medical devices at BIOMEDevice Boston, April 13-14, 2016. |
Brian Buntz is the editor-in-chief of Qmed. Follow him on Twitter at @brian_buntz. Chris Newmarker is senior editor of Qmed. Follow him on Twitter at @newmarker
Like what you're reading? Subscribe to our daily e-newsletter.
About the Author(s)
You May Also Like

.png?width=300&auto=webp&quality=80&disable=upscale)
