Edwards Faces Class I Recall for Catheter Tips
November 5, 2013
A recall of deformed bypass catheter tips from Edwards Lifesciences has been given Class I status by FDA.
|
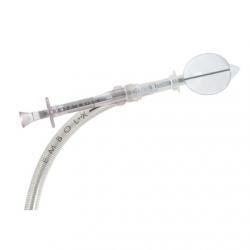
The EmbolX is one of Edwards' many cardiovascular device products. |
According to the FDA notice, the Embol-X Glide Protection system is a specialized access catheter that can be used to capture tissue fragments and blood clots during bypass operations. The cannula tips that come with the device are deformed, increasing the risk of embolization and separation during insertion or removal procedures.For its part, Edwards Lifesciences has requested that its customers isolate the affected cannulae and return them to the company for replacement. Only the cannulae on each Embol-X catheter are affected by this problem.While there haven't been any reports of injury or death associated with the devices, the use of devices with the damaged cannulae could lead to serious patient injury or death according to FDA.Affected devices were manufactured at the company's production facility in Draper, UT. This is the same facility that received an FDA warning letter in early 2013. The latest recall doesn't state if these two issues are related.The company also could see increasing competition next year in the transcatheter aortic valve replacement market, as Medtronic expects to have its CoreValve device approved by FDA. In October, JP Morgan analyst Michael Weinstein downgraded the firm's stock from 'neutral' to 'underweight,' reducing the price target from $64.00 to $62.00.
About the Author(s)
You May Also Like

.png?width=300&auto=webp&quality=80&disable=upscale)
