Boston Scientific Faces Subpoena over Defibrillators
May 9, 2014
Federal investigators apparently are still scrutinizing the way Boston Scientific handles its defibrillator business.
|
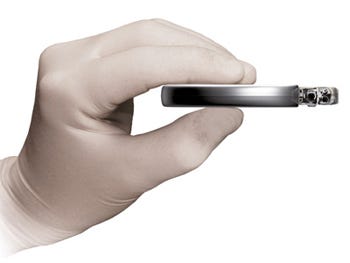
Boston Scientific touts the Teligen as the smallest, thinnest high-energy ICD in the world. |
The Natick, MA-based medical device giant recently disclosed in an SEC filing that the U.S. Department of Health and Human Services, Office of the Inspector General, served it with a subpoena on May 5.
The subpoena, according to Boston Scientific, "seeks information relating to the launch of the Cognis and Teligen line of devices in 2008, the performance of those devices from 2007 to 2009, and the operation of the Physician Guided Learning Program." The company says it is cooperating with the request.
Boston Scientific has boasted that the Cognis is the smallest (32.5 cc) and thinnest (9.9 mm) high-energy cardiac resynchronization therapy defibrillator. And at 30.5 cc in size and 9.9 mm in thickness, the Teligen is touted as the smallest, thinnest high-energy ICD in the world.
Both products, though, have had their share of troubles. Boston Scientific acknowledged in 2012 that a rare malfunction in a transformer used in both devices had caused one death, according to a report at the time in the Boston Business Journal. The company in 2010 wrote off $1.8 billion in costs related to a month-long recall of its implantable heart defibrillators, according to a Bloomberg report.
Refresh your medical device industry knowledge at MD&M East, June 9-12, 2014 in New York City. |
Boston Scientific has also had a host of legal troubles related to defibrillators, going back to the actions of cardiac device maker Guidant Corp., which Boston Scientific acquired in 2006 for $27.3 billion in what Fortune described as the "(second) worst deal ever."
Last year, Boston Scientific agreed to pay $30 million to settle federal allegations that, between 2002 and 2005, Guidant knowingly sold defective heart devices to health care facilities that in turn implanted the devices into Medicare patients.
A federal judge in St. Paul, MN in 2011 formally convicted and sentenced Boston Scientific to pay $296 million because Guidant in the past had withheld information from the FDA regarding catastrophic failures in some of its lifesaving devices. The U.S. Justice Department said at the time: "Guidant made decisions at various junctures to conceal information from the FDA and medical professionals regarding the device failures. In June 2005, the company finally went public about the problem with information it had known for 10 months, and then only after three deaths had occurred."
Chris Newmarker is senior editor of MPMN and Qmed. Follow him on Twitter at @newmarker.
About the Author(s)
You May Also Like

.png?width=300&auto=webp&quality=80&disable=upscale)
