Wrist Fixation Device Gets Extreme Makeover
March 6, 2005
Originally Published MPMN March 2005
PROFILE
Wrist Fixation Device Gets Extreme Makeover
A lightweight, radiolucent polycarbonate is credited with enabling a design breakthrough
|
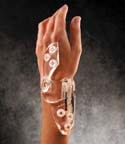
A polycarbonate resin from GE Advanced Materials helps to bring wrist fixation device technology |
When a person falls forward, his or her impulse is to cushion the fall with an outstretched hand. Bad idea (although there are no good alternatives). The impact of the body weight on the hand may cause the end of the lower arm bone to fracture just above the wrist. This fairly common injury is known as a Colles fracture.
The remedy involves immobilizing and repositioning the injured bones. Conventional wrist fixation devices, however, can add insult to injury. While they are effective, the metal rod-and-screw devices have the stylistic flair of medieval instruments. Rigid fx Orthopedics Corp. (Austin, TX) set out to give the device a makeover. To do so, the firm needed to source a clear, lightweight, and radiolucent material. It found a solution in a polycarbonate (PC) resin from GE Advanced Materials (Pittsfield, MA; www.geadvancedmaterials.com).
The Lexan HPS7 resin enabled Rigid fx Orthopedics to develop a product that has won over patients and doctors alike, according to the firm. Weighing just 1.9 oz, the ClearView Wrist Fixator has a high-tech appearance that is a big hit with patients, who prefer it to unwieldy and heavy conventional devices. Surgeons applaud the product’s translucent and radiolucent qualities. Traditional metal fixation instruments impede visibility of the injured area and obstruct x-rays. The resin from GE Advanced Materials made it possible to develop a device that affords surgeons an unobstructed view.
Another key factor in the selection of the PC resin, notes Clare Frissora, market director for healthcare at GE Advanced Materials, is its resistance to sterilization. The material provides impact resistance even after numerous autoclave cycles. It also maintains its performance properties following repeated exposure to gamma and steam sterilization.
Introduced last summer, Lexan HPS7 is biocompatible based on the ISO 10993 standard. Gamma stability is achieved by means of a proprietary nonbrominated formulation. The lipid-resistant material has a melt flow index (MFI) of 5 g per 10 minutes at 300°C/1.2 kg.
Because it is lipid resistant and withstands gamma and autoclave sterilization, the material may be suited for use in various other medical applications. The company cites dialysis components, stopcocks, luer fittings, and y sites as examples of the possibilities.
GE Advanced Materials also recently introduced Lexan HPS4 and Lexan 4404 resins, both of which exhibit desirable properties for healthcare applications.
Lexan HPS4 is a gamma-stabilized material engineered for an MFI of 10. Bromines are not used in the formulation. Biocompatible and hydrolytically stable, the material is designed to meet a range of blood-care, surgical instrument, fluid-delivery, and housing applications.
Lexan 4404 is a clear high-heat PC designed for use in devices exposed to multiple autoclave cycles at temperatures up to 134°C. Also biocompatible, it offers better dimensional stability than most common PC resins, according to the firm. Surgical instruments and medical support equipment are among the applications.
Several materials currently in the pipeline further demonstrate the company’s commitment to supporting the healthcare industry, notes Frissora. “We have a new Lexan resin that achieves improved color stability following gamma sterilization,” she says. “Another technology is under development for applications that require a combination of flow and ductility, processability, and multiple autoclave sterilization cycles that cannot be met by traditional PC grades,” Frissora adds. These materials are not yet on the market, but they are available for sampling.
Copyright ©2004 Medical Product Manufacturing News
You May Also Like

.png?width=300&auto=webp&quality=80&disable=upscale)
