The Little Devices That Could
A promising crop of miniature and easily concealed medical devices enables patients to do more with less equipment.
April 1, 2009
MDEA 2009
Making daily tasks easier for people is a simple but effective path to success. That concept wasn't lost on winners of this year's Medical Design Excellence Awards (MDEA). Patient-enabling technology was at the forefront among this year's winners. This was often executed in the form of miniature, more-convenient devices that allow users to continue with their daily lives with minimal interruption because of health concerns. These devices can fit inside a pocket, an ear, a shoe, or even a nostril.
Devices that enable the patient help “keep people out of the hospital and move healthcare to the home,” according to MDEA juror Steve Wilcox, principal and founder of the firm Design Science (Philadelphia). This is especially the case as medical devices continue to improve the quality of life for people with chronic conditions.
“The trend is to minimize the complications and compromises for the patient, while providing better compensation for the chronic condition,” Wilcox says.
Sweeter Meters and Controls
|
The UniDose can be bundled with glucose test strips to make testing easier for the user. |
For diabetics that self-test for blood glucose levels, accuracy is critical. To that end, Bionostics Inc. (Devens, MA) has developed a single-use product that enables the user to verify the performance of a blood glucose test system with a single drop of quality control solution. The UniDose glucose meter check control, a foil package containing test solution for glucose meters, is smaller than a standard business card and virtually just as thin. The easily opened control packet is made of laminated foil.
Current control products are often supplied in start-up kits for recently diagnosed diabetics, and Medicare patients receive them once every three months. But obtaining replacements can be tricky. Local pharmacies may not carry replacement controls, and they typically have to be ordered by mail. The UniDose's small size allows it to be bundled with each package of glucose test strips and to be quickly available when needed.
“If delivered together with glucose test strips, the right control solution is always readily available to comply with manufacturers' instructions to test each new set of test strips prior to use to ensure they are measuring correctly,” says Randy Byrd, vice president and CTO of Bionostics. “Improved compliance should improve outcomes.”
With light pressure applied, the UniDose releases a small drop—less than 100 μl—of the colored solution. A glucose test strip can be touched to the solution to perform the control check. The liquid that pools out from the UniDose is a viscosity-adjusted solution made red with food-grade artificial color (to simulate the blood collected from sticking one's finger).
Test solutions for glucose meters have been troubled by contamination problems. Up to this point, a glucose control solution has typically been dispensed from a plastic vial, with a drop of the solution being deposited onto a target area of the test strip. But the sample size required by the test strips has become increasingly small. As a consequence, a large drop of the control solution can flood the test strip, which can damage the meter or result in variable measurement.
“Standard packaging of control solutions is in a dropper bottle. With the dropper bottle design there is the possibility for sample carryover and contamination of the control solution,” says Mark Vreeke, an MDEA juror and senior partner at the Houston-based consulting firm Rational Systems LLC. “The single-use design eliminates the control solution as a potential source of error or failure when verifying meter and strip performance.��”
|
The True2go, which fits on the vial of test strips, is a reusable meter that twists on and off the vial with a quarter turn. |
Enabling diabetic patients to enjoy a more carefree lifestyle is taken even further by the True2go portable glucose monitor by Home Diagnostics Inc. (Fort Lauderdale, FL). The True2go measures about 1.7 × 1.5 × 0.9 in. and weighs less than an ounce. It can be used as a stand-alone device or can be attached to a vial of glucose test strips. When Home Diagnostics developed the system, it set out to create a whole-in-one device that mated the meter and the strips in a lightweight, portable package. Such a design enables a diabetic to reduce the number of items necessary to tote around for testing. This can be especially helpful while traveling, during which time diabetics sometimes have a harder time adhering to a set testing schedule. The engineering challenge was to design a meter that could be easily affixed to and removed from the strip vial. The result? A reusable meter, protected by a durable polycarbonate housing, that twists on and off the vial with a quarter turn.
“The True2go fills a niche between the traditional blood glucose monitor and the bulky, costly integrated systems,” says Brent Modzelewski, director of engineering at Home Diagnostics. “What diabetics need and want is a small portable device that reduces the burden of daily testing. The quick test time and very low sample volume requirements also reduce the overall impact of testing on the user.”
Another convenient aspect for the user is the lithium battery's durability. More than 3500 readings can be performed before it needs to be replaced.
The meter also has somewhat of a brain. It automatically powers on and self-calibrates when the user inserts a test strip. Its technology allows it to read an electrochemical marker in the glucose control solution—meaning it can tell whether a blood sample or a control sample is on the strip. When the user finishes the glucose test, the True2go stores the result in its memory with a marker that indicates whether sample was blood or a glucose control solution. Once the user presses the eject button, the strip comes out and the meter automatically powers off. The process offers fewer steps for the user to remember, thereby reducing the opportunity for error.
“The True2go glucose monitor offers improvement in patient convenience. It integrates both the meter and strip vial into a single package that is only slightly larger than the strip vial itself,” Vreeke says. “This dual function configuration reduces the [number of] elements that must be assembled to perform a glucose test.”
Taking Back the Night
|
A noninvasive valve design is the key feature for the Provent device, which harnesses its user's own breathing to create positive airway pressure. |
Reducing the number and size of parts necessary for treatment has proven a good route to follow. Ventus Medical Inc. (Belmont, CA) yielded to that approach with the Provent professional sleep apnea therapy device. Obstructive sleep apnea is a condition in which a person stops breathing during sleep due to an airway blockage (often occurring from soft tissue at the back of the throat). The National Institutes of Health estimates that more than 12 million Americans have the condition, putting it on par with adult diabetes. Treatment often consists of a continuous positive airway pressure (CPAP) machine, tubing, and headgear fitted to each patient. Treatment is more effective in the lab setting compared with the home environment, because even though noncompliance is a health risk, many sufferers of sleep apnea are reticent to wear such a contraption during sleep. In addition, CPAP users bemoan the device's lack of portability.
“The goal was to create a radically different treatment for obstructive sleep apnea—providing clinically proven efficacy in a convenient device that the user (and the user's partner) could barely see, and that users would actually wear on an ongoing basis,” says Rajiv Doshi, founder of Ventus Medical.
Getting the right material was a challenge. The company searched for a gentle, hypoallergenic adhesive “that was easy on the skin but that was strong enough to maintain an airtight seal overnight on a variety of skin types,” according to Doshi. Ventus pored over dozens of adhesive products to find one that provided the necessary balance.
The disposable device also uses a noninvasive valve design that harnesses its user's own breathing to create positive airway pressure—a method that eliminates the need for the tubing, mask, and machine. Plus, a device that fits in the nasal passage can't be too hard to travel with.
“I liked the fact that patients did not have to carry an electronic device and that [the Provent] was disposable and easy to transport,” says MDEA juror Denise Korniewicz. She is a professor and senior associate dean for research at the University of Miami School of Nursing and Health Studies. “I think that patients who are currently ‘noncompliant' may [become] compliant because they would not have to carry a machine around with them—nor would they have to feel claustrophobic.”
Users place the device just inside the nostrils, where it is held in place by a hypoallergenic adhesive. During inhalation, the valve opens to allow minimally obstructed airflow. During exhalation, the valve closes and air passing through the nose is then funneled through two small air channels. Ventus also succeeded in creating a quiet valve mechanism (i.e., one that didn't flutter or create other sounds that could disrupt sleep) that would open and close based on the direction of airflow.
Provent's “ease of use, disposability, and simple technology” add to its appeal, Korniewicz says, making it a promising alternative for sleep apnea patients.
Happy Feet
|
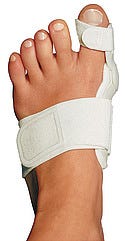
The Bunion Aid's corrective strap around the big toe helps adjust alignment. |
As some devices move toward the miniature, others are shifting toward the invisible—to observers, at least. For people with bunions, there have been few effective alternatives to having surgery to correct deformities. Pain may prevent them from walking comfortably, but toe spacers and bunion cushions only provide limited relief. Alpha Orthotics Corp. (Tiburon, CA) realized that feet need attention too, and that's what they'll get from the company's Bunion Aid, which fits inside the user's shoe. It allows for natural mobility of the base joint of the big toe, which in turn allows users to walk without altering their gait.
Axel Krauss, an automobile mechanic who reinvented himself as an orthotics designer, came up with the idea for the Bunion Aid after trying to help an 8-year-old girl who suffered from painful toe misalignment. The device uses a dynamic splint set to the medial border of the foot to address mild to moderate bunion deformities. The brace's hinge aligns with the first metatarsophalangeal joint.
“It is surprisingly comfortable and easy to put on, and it actually fits inside the shoe,” Wilcox says. “The central idea is to exert spring force on the big toe in the opposite direction from the dis-tortion caused by a bunion, i.e., inward.”
The design wasn't without challenges. For example, the rigid portion and the hinge of the splint had to be strong enough to sustain forces of 125% of a person's body weight. Also, the straps of the Bunion Aid had to be designed so that they did not introduce torsion or twisting of the big toe.
“[The Bunion Aid] is nicely engineered and able to maintain the position of the [big] toe in relation to the rest of the foot,” Vreeke says.
In this case, a simple design makes the splint effective. A strap near the middle of the foot is used for fixation, and a corrective strap around the big toe helps adjust alignment. A pad on the bottom of the foot supports the foot's arch, and the hinged splint has a pad to relieve pressure on the foot. The joint hinge and the slim profile of the splint enable the user to fit the device inside a shoe and walk normally. Alpha created the device in hopes that it would serve as a more conservative treatment (i.e., helping users avoid or postpone invasive surgery to correct misalignment).
“Sometimes the deviation doesn't have to be that large and people can have a lot of pain,” says Alice Flaherty, a physical therapist and clinical consultant for Alpha Orthotics. “Podiatrists will come back to us and say, ‘I have a patient who is a nurse who can't take five or six weeks off to have surgery.'”
Music to My Ears
|
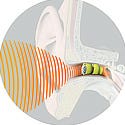
The Lyric, which is invisible to observers, enables its users to hear better due to its proximity to the tympanic membrane. |
A concept that's good for the foot may also be good for the ear. The Lyric hearing aid, manufactured by Newark, CA–based InSound Medical Inc., is another device that cannot be seen when it is worn. It is placed inside the ear canal and worn 24 hours a day, with a battery that offers four months' worth of power before it needs to be replaced. Users can shower, exercise, and talk on the phone without having to remove or adjust it.
The invisible design was critical for two reasons. First, “more people would actually wear it and get help for their hearing loss,” according to Susan Whichard, InSound's vice president of marketing. “It really affects our communication and cuts you off from many things in our world. It's an underserved market.”
In addition, the Lyric's placement within the ear allows for better hearing for its users. “Scientifically, we know that to put a hearing aid all the way in the ear canal would afford the most natural listening,” Whichard says. “To have the microphone that picks up the sound, as well as the speaker that delivers the sound, both in the ear canal allows the outer ear to naturally collect the sound just like people with normal hearing.”
Previous completely-in-the-canal (CIC) hearing devices have been hindered by an inability to fit the ear (fitting is often done by making impressions of the deep ear canal). InSound developed a sizing and placement system that circumvents the hassles of ear impressions. A trained professional places the device 4 mm away from the tympanic membrane, which transmits sound inside the ear.
“Because of its proximity to the tympanic membrane, less-complicated signal processing is required, with the pinna (outer ear) naturally focusing sound toward the Lyric,” observes juror Gail Baura, a professor at the Keck Graduate Institute (Claremont, CA).
The Lyric's housing is covered with proprietary coatings that protect it from potentially harmful elements in the ear such as moisture, debris, and ear wax. Another challenge of previous CIC hearing devices was comfort, mostly due to the sensitive and inflexible bony portion of the ear canal. To address this, the Lyric is held in place inside the ear by two biocompatible soft foam seals.
“I liked the fact that the device could be implanted and that patients would be more compliant to using the hearing device since no one would be able to see it,” Korniewicz says. “Second, the antimicrobial material is a key feature so that users do not have to worry about ear infections.”
To further enable the user, a magnetic adjustment tool allows the user to adjust the volume, turn it on and off, or put it in sleep mode. There are also loops for self-removal in case of an emergency.
Conclusion
|
|
The influx of small, portable devices into the marketplace is par for the course in today's medical climate. It makes sense that as healthcare becomes more expensive, making patients independent is one way to reduce the costs. Juror Molly Story, president of Human Spectrum Design LLC (Santa Rosa, CA), wonders whether baby boomers have something to do with it.
“The baby-boom generation is aging and is not very tolerant of the process, so they want devices to make their lives better with the least possible inconvenience. The demand is there and the market follows,” she says.
Indeed, many of the medical devices that earned a Medical Design Excellence Award reflected a shift of power from the physician to the patient. Smaller devices. Less equipment. More convenience. As users demand more from their devices, they are also enabling themselves to do more with less.
Lawrence Lloyd is associate editor of MD&DI.
Copyright ©2009 Medical Device & Diagnostic Industry
About the Author(s)
You May Also Like





.png?width=300&auto=webp&quality=80&disable=upscale)
