Peripheral Vascular Stents Present Billion-Dollar Opportunity
November 1, 2006
NEWS TRENDS
|
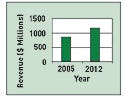
Figure 1. Revenue growth in U.S. peripheral stent and stent grafts. |
Coronary stent manufacturers could benefit by tailoring their devices for use in the peripheral vascular region. According to a Frost & Sullivan (Palo Alto, CA) report, peripheral vascular disease is an underserved market that presents a billion-dollar opportunity for stent manufacturers. Increased awareness and reimbursement of preventive screening measures that lead to the use of peripheral stents will also push growth in the market.
The U.S. Peripheral Vascular Stent and Stent Grafts Market analysis reports the sector had revenues of $855.8 million last year. As these stent procedures become the gold standard for treating peripheral vascular disease, revenue is projected to hit $1.153 billion by 2012 (see Figures 1 and 2). The devices included in the report are abdominal aortic aneurysm stent grafts and carotid, femoral-popliteal, renal, biliary, and iliac stents.
In general, the success of coronary stents has triggered interest in peripheral stents, says Venkat Rajan, industry analyst for the medical device team at Frost & Sullivan. Both large and small companies should be able to take advantage of the market opportunity. However, large manufacturers, such as Boston Scientific and Johnson & Johnson, could have the upper hand because of costly development and product familiarity. “When interventional and endovascular surgeons are picking a device to use, they tend to prefer the most familiar one,” says Rajan. “In the renal, iliac, and biliary market, which is a little more mature than the other ones, you see a lot of off-label device use.”
|
Figure 2. The U.S. peripheral stent and stent graft market revenue forecast, 2004–2006. |
According to Rajan, the carotid stents have the highest growth potential. Previously when stenting procedures were performed, there was a risk that dislodged emboli could travel into the microvasculature of the brain and cause a rupture. The development of embolic protection filters and capture devices has solved this problem and has prompted growth in the sector. When the device is used in conjunction with the filter, it captures the emboli.
The acquisition by Abbott Laboratories of Guidant's vascular business makes it the main player in this market. Last year, it received FDA approval for the Xact Carotid Stent and Emboshield Embolic Protection System. The company then gained Guidant's Acculink carotid protection system and Accunet embolic protection system earlier this year. Boston Scientific also has an agreement with EndoTex Interventional Systems Inc. (Cupertino, CA) to use its Filterwire embolic protection device with EndoTex's carotid stent. Rajan adds that a couple of companies are poised to enter this market soon.
New methods of screening for abdominal aortic aneurysms (AAA), a condition labeled a silent killer, will push the use of AAA stent grafts. “There was a lot of interest when these devices were first introduced, but then the market leveled out a bit,” says Rajan. “Improved screening and approval from Medicare on these procedures is spurring usage of these devices. The imaging side has also improved device placement and accuracy.” Major AAA stent graft market players are W.L. Gore & Associates Inc. (Newark, DE), Medtronic Inc. (Minneapolis), Cook Inc. (Bloomington, IN), and Endologix Inc. (Irvine, CA).
Another factor that could affect peripheral vascular market growth is next-generation devices with drug-eluting capabilities. The concept for devices that treat coronary arteries could be transferred to the peripheral arteries. However, there are different design challenges when dealing with the peripheral vasculature.
“It's completely different than a blockage in the coronary artery,” says Rajan. “In the lower limbs, occlusions can be longer than 20 cm. They're also located between muscles and bones, so there's a lot of movement, which can cause stent displacement or fracture.” Peripheral vascular devices need to be more flexible and more versatile than their counterparts in the coronary market. It's possible that experienced stent manufacturers could take advantage of this prospect in the future.
Copyright ©2006 Medical Device & Diagnostic Industry
About the Author(s)
You May Also Like