IAF Hopes Global Medical Device Trade Will Improve with Accredited ISO 13485 Certification
One of the things that never really changes in the field of medical device compliance is how we look at solving our trade problems. For decades we heard, “Accepted once, accepted everywhere. Harmonization is the answer.” But after the collapse of the Global Harmonization Task Force (GHTF) and much food fight–style banter at the World Trade Organization (WTO), the world has ended discussions with too little progress in serving up solutions that genuinely improve medical device trade.
The problem with solving trade within the public health protection sphere is the sincere obligations that regulators have to protect their own people. As a result, what happened at the GHTF can be seen most clearly as a diaspora away from harmonization, as each nation creates versions of the harmonized guidance. In the end, the abandonment of trade interests has compromised national health concerns. Nowhere has this become more obvious than in divergence in the area of regulating the quality management systems (QMSes) of medical device manufacturers.
Following the strict enforcement of the Medical Device Directive in Europe in 1998, there was an explosion of demand for QMS-based certifications. ISO 9001, EN 46001, and 13485 formed the initial base for CE marking and the FDA Quality System Regulation (QSR). The two largest trading regions set in motion the example to which the rest of the world’s regulators would eventually look to harmonize their own medical device regulations. Canada, Japan, Brazil, and others have since followed, creating their own national variations of this new approach. “What happened to ‘accepted once, accepted everywhere?’” was the cry from industry. Each country created national versions.
Countries that developed their own QSRs discovered serious complications with this approach. Regulatory authorities, including FDA, did not have the resources to inspect the thousands of manufacturers scattered all over the world. Language and cultural barriers prevented even the most experienced auditors from knowing what was being done in foreign factories, even while they were there. In smaller countries where a national QSR was enacted, healthcare professionals soon discovered it was impossible to gain legal access to devices that were widely accepted by larger modernized regulatory systems. The phrase was coined in one smaller country, “A barrier to trade is a barrier to care,” and a system had to be created to legally circumvent its own QMS regulation.
|
Some of the most prevalent diseases in the world are treated by medical devices manufactured by small and medium-sized companies. Where the expense of supporting another nationalized regulatory audit to ISO 13485 is felt to be too great a burden, manufacturers have been known to withdraw their medical devices from these smaller countries, causing their healthcare systems to suffer. (U.S. Navy photo by Mass Communication Specialist 1st Class Brian A. Goyak/Released) |
Why Harmonization Alone Could Never Work
Effective healthcare protection concerns could never be solved through harmonization. Regulators need confidence that auditors are competent. Regulators need access to the results of the audits, whenever they want them. Regulators also need a system sized for a global economy and that can address language and cultural barriers without compromise. This is not something a standard can do or enforce. Regulations will always require some badge-wearing-style enforcement if they are to be effective. These have been the core obstacles to unlocking medical device trade’s Holy Grail of “accepted once, accepted everywhere.” It can only be resolved using an international organization that has the structure and capacity to support regulatory enforcement worldwide.
Supporting Credible Certification Worldwide
The International Accreditation Forum (IAF) contains the largest assembly of government-recognized accreditation bodies in the world. Early GHTF documents actually refer to IAF guidance. Since then, some of the old IAF guidance has been developed into ISO standards for accreditation that exist today as ISO 17021 and ISO 17011, which describe how conformity assessment bodies (CABs) and accreditation bodies (ABs) must operate. With membership at the IAF growing to more than 60 nations, there is no greater assemblage of nations tied together in the field of accredited certification for quality management systems. Not only does the IAF have arrangements to apply accreditation requirements systematically and worldwide, it also has teeth. The IAF can enforce accreditation activities by pressing its members to remove accreditation marks from violative CABs that ignore accreditation requirements. This strengthens the IAF’s utility as a body capable of assisting regulators in managing medical device certifications worldwide. Although manufacturers may be yawning at the idea of making regulators happy, the result of the IAF’s effort lends itself to realizing the vision statement of the IAF: “Certified once, accepted everywhere.”
Accreditation Requirements for ISO 13485—The IAF Mandatory Documents
The IAF mandatory documents (MD8 and MD9) were completed in July 2011. All accredited ISO 13485 certificates will be issued under the controlled implementation of these two IAF mandatory document requirements, beginning in July 2012.
MD8 applies specific additional requirements for the application of ISO 17011, which describes how ABs are to operate. MD9 does the same for the application of ISO 17021 for CABs. It’s worth noting that both the ABs and CABs have been operating under the two base ISO standards for the past few years but without the much needed guidance related to medical devices.
Among other things, the two IAF mandatory documents establish requirements for auditor competencies related to specific medical devices processing and broader knowledge of risk management standard ISO 14971 and even existing regulations.
|
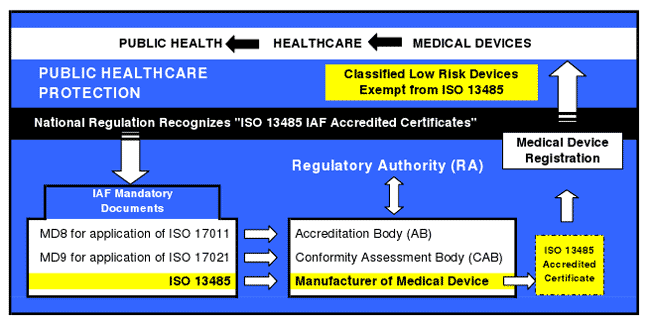
This chart shows the interrelationship between IAF mandatory documents for accredited certification to ISO 13485 and a harmonized medical device regulation, including support of a national healthcare infrastructure. |
What Will it Cost?
Industry will not likely experience any differences in the ISO 13485 assessment costs. The thinking is that certain cut-rate certifications will begin to fade away. These types of certification have already diminished greatly, as most ISO 13485 certifications are issued by CABs providing many other highly regulated QMS audits manufacturers must get for Europe, Canada, Japan, and other regions where application of ISO 13485 is now essential for regulatory compliance. The benefits will far outweigh the costs.
The IAF and ‘Certified Once, Accepted Everywhere’ for ISO 13485
In addition, the IAF recently voted to accept ISO 13485 as a subscope to the largest international medical device trade agreement in the world, the IAF Multilateral Recognition Arrangement (MLA). The IAF signatory members, most of which work adjacent to their ministries of health, will agree to accept an ISO 13485 certificate accredited by any other IAF member signatory. All IAF member signatories will agree to apply the new accreditation requirements and must agree to regular surveillance by a special IAF MLA management committee, which will assess the extent to which each member is following and enforcing the new mandatory requirements for accreditation certifications issued for ISO 13485.
|
The IAF MLA Mark will eventually appear on accredited ISO 13485 certificates. |
Improving Healthcare Worldwide?
The vision of the IAF ISO 13485 working group has always been “to provide opportunities to develop medical device regulations, while maintaining access to safe and effective healthcare technologies.” The IAF has plans to publish another informative document, which will describe how a harmonized medical device regulation might be developed, with information on how medical devices are classified. It also cautions against requiring certification to ISO 13485 where low-risk devices are concerned. The IAF program was developed using guidance and input provided by regulators and is mindful that sustaining access to medical devices is just as critical as screening them through a balanced, risk-based approach.
Openness and Communication
Regulator access to audit reports is part of the marriage. The IAF mandatory document MD9 requires release of audit reports to regulators that formally recognize ISO 13485 in their medical device regulations. Regulators that do not formally recognize ISO 13485 in their regulation will not be able to obtain audit reports from the CABs. There are also strengthened requirements for communication with regulatory agencies. Regulators have always been expected to use ISO 13485 for regulatory purposes, just as the title of the standard suggests. The IAF working group thought it made good sense to add these features, so regulators would not have to create their own system for accreditation.
By giving regulators an instrument to improve public health at the national level using an international approach, we can now improve healthcare worldwide. The Holy Grail that has eluded industry also had to sustain life on a global scale. The new IAF accreditation system will offer opportunities for developing countries to improve healthcare protections without risking loss of access to devices they need. Most smaller economies import 90% or more of their devices. Interrupting this flow has to be done with great care. Using a nation’s accreditation body connected with 60 other countries at the IAF is victory for healthcare protection and trade.
Solving international trade issues is always about recognition. Harmonization is merely a path. Credible, believable, trustworthy, transparent certifications are worthy of that recognition. In July 2012, harmonization will meet recognition. Medical device trade will change for the better.
Grant Ramaley has been the cochairman of the regulatory affairs and standards committee for the Dental Trade Alliance for more than 10 years and is a current coconvener for the International Accreditation Forum’s working group for ISO 13485. Ramaley is also employed as the director of regulatory affairs at Aseptico Inc. (Woodinville, WA).
About the Author(s)
You May Also Like