An Uncertain Path
A new survey indicates industry’s desire for more detailed guidance for developing and commercializing combination products.
INDUSTRY ASSOCIATIONS
|
(click to enlarge) |
Over the past few years, the realm of combination products has undergone dramatic growth and change. Many start-up companies have ventured into the converging world of medical devices, pharmaceuticals, and biologics, and several of industry's largest players are also grappling with the challenges posed when two or more FDA-regulated articles are joined together.
In 2007, FDA's Office of Combination Products (OCP) underwent several leadership changes. Meanwhile, the much-anticipated good manufacturing practices (GMPs) and adverse-event reporting regulations for combination products continued to wind their way through the agency's internal review processes. Now, with Thinh Nguyen in place as the new permanent director, OCP seems poised to make new and exciting headway in its quest to further develop and refine the regulatory landscape for combination products.
Against this backdrop, in late 2007, the Combination Products Coalition (CPC; Washington, DC) began to plan its 2008 advocacy agenda. As it did, the organization's diverse group of member companies identified a need to step back and take the industry's pulse on existing combination product policies and guidance. The organization's goal in doing so was to ensure that it remained focused on its mission of developing and advocating improved policy positions on regulatory issues affecting combination products, which necessarily cut across multiple diverse industries.
With that goal in mind, the CPC members developed and sponsored an online survey designed to gauge industry priorities for guidance and rulemaking activities in the realm of combination products. The results of the survey—which was conducted in December 2007 with the assistance of survey software from the Regulatory Affairs Professionals Society—are being used to develop CPC's 2008 policy agenda and offer input on policy development priorities to OCP. This article summarizes the results of the survey.
Survey Scope and Methodology
The CPC survey was designed to evaluate participating manufacturers' demographics, their satisfaction with existing combination product regulatory guidance, and their opinions as to topics on which more or better guidance is needed. Participants ranked potential regulatory guidance topics according to perceived importance and then answered questions related to the type of guidance they would like to see and why they thought such guidance was needed. Throughout the survey, respondents were encouraged to elaborate on their answers in free-form comment boxes.
The survey was distributed widely among pharmaceutical, medical device, and biologics manufacturers with the help of several trade groups and industry publications, including MX magazine. Respondents completing the online survey were allowed to remain anonymous, although they could submit optional identifying information. In order to avoid having a single company or industry segment disproportionately represented, respondents were asked to complete only one survey per organization. However, due to the anonymity provided by the survey software, companies' adherence to this request could not be confirmed. Individuals completing the survey were asked to collaborate with colleagues at their company to provide a comprehensive view of their organizations' activities.
Demographics
The first section of the CPC survey revealed the following respondent characteristics and experiences.
Primary Product Focus. The medtech sector was particularly well-represented among survey respondents, with 78% of the survey's 32 participants indicating that their primary product focus was medical devices. Five of the 32 companies (16%) indicated that their primary product focus was pharmaceuticals, while only two companies (6%) said that biological products were their primary product focus.
|
Figure 1. (click to enlarge) |
Annual Sales. Survey respondents represented a wide range of sizes—from start-up companies to manufacturers with more than $1 billion in annual domestic combination product sales (see Figure 1). However, the majority of respondents indicated they were either start-up companies or had less than $100 million in annual U.S. sales of combination products—which is not surprising given the relatively recent advent of combination products.
|
Figure 2. (click to enlarge) |
Combination Product Experience. In order to stratify responses by experience level, the survey asked respondents about their familiarity with developing and commercializing combination products. Participants were asked to rate their experience on a scale from no experience to extremely experienced. Nearly half (47%) said they had a moderate level of experience with combination products, which was defined as having commercialized, developed, or licensed and marketed at least one combination product. Slightly less than a quarter of respondents (22%) said they had low experience, and another 22% described themselves as extremely experienced. Low experience was defined as having one or more combination products in the beginning stages of development. Extremely experienced was defined as having commercialized, developed, or licensed and marketed several combination products. Only three survey participants (9%) said they had no experience at all with developing or commercializing combination products.
|
Figure 3. (click to enlarge) |
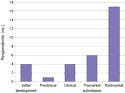
The survey asked respondents to indicate the stage of their most-developed combination product. The majority of respondents had at least one product in the postmarket stage (see Figure 2). However, every stage of development was represented among respondents. Also, the survey gauged companies in regard to the total number of combination products that they had developed and brought to market (see Figure 3). The majority of respondents had produced between zero and three products, although five companies indicated that they had more than 10 combination products on the market.
|
Figure 4. (click to enlarge) |
The survey also required companies to categorize their combination products in the same way that OCP categorizes them. Respondents were allowed to select as many categories as needed (see Figure 4).1 The majority of respondents indicated that they were developing a combination product composed of a device that is coated or otherwise combined with a drug. Other top responses included prefilled drug-delivery devices or systems, devices coated or otherwise combined with a biological product, and cross-labeled products. The large number of products with device components is not surprising given the number of medtech companies completing the survey. Also, the types of products represented in the survey were generally proportionate to the number and type of combination products that undergo FDA review.
Satisfaction with Existing Guidance
|
Figure 5. (click to enlarge) |
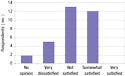
The second major section of the survey inquired about respondents' satisfaction with existing guidance sources, including both FDA and non-FDA sources, such as trade associations, consultants, and legal counsel. The latter sources were included as means of assessing the totality of existing guidance. More than half of the respondents (56%) indicated some level of dissatisfaction with existing guidance, saying they were either not satisfied or very dissatisfied (see Figure 5). Although 12 of the 32 participants said they were somewhat satisfied with existing guidance, no company indicated that it was very satisfied with existing guidance on combination products.
All three participants who said they had no experience with combination products indicated they were dissatisfied with existing guidance. Further, only one of the six respondents at the premarket submission level of product development indicated any level of satisfaction. In addition, only one of the five pharmaceutical companies said they were satisfied with existing guidance.
On a more granular level, some respondents offered additional comments about their satisfaction with existing guidance. For example, one respondent commented on the need for additional detail in FDA guidance, noting that "part of the problem with existing guidance documents is that they are at the 40,000-foot level, and there needs to be more guidance at the 10,000-foot level." Other respondents commented on specific areas where they were dissatisfied with existing guidance, such as device change control issues and the lack of detail on how pharmaceutical requirements apply to combination products.
Topics for FDA Guidance
In gauging the need for regulatory guidance on specific topics, the survey offered participants a list of 17 distinct regulatory topics and asked them to select the five topics on which they believe combination product guidance is needed most.
Weighted rankings were determined by assigning selected topics a point value from 1 to 5; a topic ranked first received a point value of 5, a topic ranked second received a value of 4, and so on. When weighted, the number-one topic was clinical studies (see Table I). In second place was GMPs—a subject on which a draft proposed rule and a well-known written guidance document do exist.2
Weighted Rank |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
11 |
12 |
13 |
14 |
Table I. Respondents' ranking of 17 regulatory guidance topics related to combination products. Each respondent selected five topics on which they believe combination product guidance is needed most. Weighted rankings were determined by assigning selected topics a point value from 1 to 5; a topic ranked first received a point value of 5, a topic ranked second received a value of 4, and so on. |
|
Figure 6. (click to enlarge) |
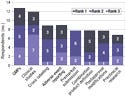
When ranked by the raw number of responses, clinical studies and GMPs switched places as the top two topics, with GMPs slightly edging out clinical studies in terms of the number of respondents ranking those subjects in their top three priorities (see Figure 6).
All companies ranking GMPs as their top priority were self-described as moderately experienced combination product companies with less than $100 million in annual domestic combination product revenue. Nearly 80% of companies that ranked GMPs as either their first or second priority were moderately experienced.
Slightly more than a third of companies (37.5%) ranked clinical studies as one of their top-three priorities. Nearly 60% of respondents placed the topics of clinical studies or preclinical research among their top-three priorities. Research (either clinical studies or preclinical research) seemed to be important for device companies in particular. Of the participants who put a research topic among their top three priorities, only two were not device companies.
A number of additional topics were cited as being important to many survey participants. These included adverse-event reporting, cross-labeling, postapproval modifications, the definition of a combination product, and premarket submissions. In addition to the priority rankings, the following observations were made in regard to respondent guidance priorities.
Not surprisingly, most companies ranking adverse-event reporting in their top three were in the postmarket stage of development (eight of nine respondents). This topic was not rated highly by companies with no or low experience in combination products.
Also not surprisingly, nearly all companies ranking postapproval modification issues as a high priority were in the postmarket stage.
Combination product definition seemed to be a concern among all types of device companies, although no drug or biological company ranked this topic as a top-three priority. One possible explanation is the ambiguity surrounding a device becoming a combination product by virtue of cross-labeling with a drug or biological product.
As priorities, cross-labeling and premarket approval submissions remained fairly consistent across all company sizes, development stages, and experiences.
Type and Reason for Guidance
After respondents ranked their priorities for guidance topics, the survey requested additional information about the type of guidance they thought was needed on those topics, and why. Participants could select more than one type of guidance for each subject.
|
Figure 7. (click to enlarge) |
For all ranked guidance topics, the majority of respondents indicated that they would prefer a traditional, written guidance document as a resource (see Figure 7). Several respondents also indicated that, in addition to a traditional guidance document, they would like to have either a question-and-answer or frequently asked questions document, documented case studies and examples, or both types of resources. Further, in some cases, respondents would also like FDA to conduct a public meeting or workshop on a particular topic. However, most respondents preferred guidance in a written format instead of guidance given orally, such as at a meeting.
The survey also asked participants to indicate why they thought guidance was needed for their top five topic priorities. Again, respondents could select as many reasons as they thought applied. The reasons from which they could choose were as follows.
Guidance doesn't exist but should be developed.
Existing guidance is inadequate (unclear, too general, etc.).
Existing guidance is inappropriately burdensome.
Existing guidance is needlessly complex.
Existing guidance conflicts with another.
Other (specify).
|
Figure 8. (click to enlarge) |
Results were peppered across all choices. However, the first two choices—guidance doesn't exist or guidance is inadequate—were by far the most frequently selected reasons (see Figure 8). Overall, the results suggest that industry wants more guidance related to combination products.
Conclusion
A few key takeaway messages were evident in the results of the CPC survey. Overall, industry members would like additional guidance—and in more detail—on combination product issues. Among the survey's limited sample size of manufacturers, clinical studies and GMPs represent top priorities for combination product guidance. Several other topics were ranked as second-tier priorities. For many of these issues, FDA and OCP have issued some preliminary guidance, either written or oral.
CPC has presented the survey results to OCP so the agency can consider the data when establishing its priorities. CPC will also continue its role in generating policy ideas for the agency to consider.
References
1. “FY 2006 Performance Report to Congress for the Office of Combination Products as Required by the Medical Device User Fee and Modernization Act of 2002,” (Rockville, MD: OCP, FDA, 2007); available from Internet: www.fda.gov/oc/combination/report2006/OCP%20Performance%20FY2006%20Final%20HHS%20Clearance.pdf.
2. “Guidance for Industry and FDA: Current Good Manufacturing Practice for Combination Products, Draft Guidance,” (Rockville, MD: OCP, FDA, 2004); available from Internet: www.fda.gov/oc/combination/OCLove1dft.html.
Copyright ©2008 MX
About the Author(s)
You May Also Like






.png?width=300&auto=webp&quality=80&disable=upscale)
.gif?width=300&auto=webp&quality=80&disable=upscale)
.png?width=300&auto=webp&quality=80&disable=upscale)