Using Nano-TMA to Rapidly Detect Environmental Stress Cracking of Polyurethane
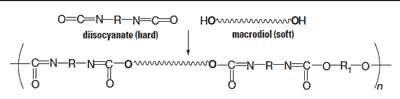
Polyurethane (PU) is widely used in medical implants such as valves, catheters, and pacemaker leads. It offers both high flexibility and strength, owing to its structure, which consists of alternating hard and soft segments.1 The hard segments aggregate and form crystalline domains, increasing the percentage of the soft domain results in a corresponding change in the polymer’s softness.
Polyurethane (PU) is widely used in medical implants such as valves, catheters, and pacemaker leads. It offers both high flexibility and strength, owing to its structure, which consists of alternating hard and soft segments.1 The hard segments aggregate and form crystalline domains, increasing the percentage of the soft domain results in a corresponding change in the polymer’s softness.
|
Figure 1. Structure of polyurethane. |
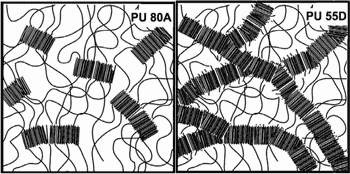
In the 1980s, pacing leads were made from PU 80A, a relatively soft version of the polymer that exhibited a high in vivo failure rate. The problem with PU 80A is that when implanted, it attracts foreign-body giant cells that attempt to digest it, releasing lysozymes and free radicals in the process.2 The resulting degradation process cleaves the PU soft segments, causing the material to embrittle and crack.
|
Figure 2. Representation of microdomain for pellathane 80A and pellathane 55D. |
For the last 20 years, pacing leads have been made with PU 55D, a relatively hard polyurethane that has proven to be reliable for this application. Though the material has a successful track record, it is still prudent to test each product made from it using accelerated aging reliability studies. The main concern with PU 55D is that a strong localized tensile stress combined with a highly corrosive environment can lead to a type of polymer degradation known as environmental stress cracking (ESC).
Simulating Degradation in the Lab
|
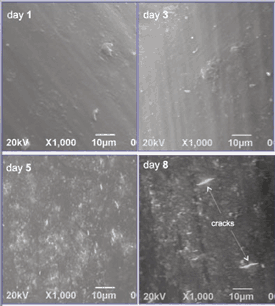
Figure 3. SEM images of PU 55D surface after one, three, five, and eight days of soaking. |
In vitro, this degradation can be emulated by submerging the sample in a solution of cobalt chloride and hydrogen peroxide (0.1 M CoCl2 and 20% H2O2) at 37°C.4 Cobalt was found to catalyze the oxidation reaction by hydrogen peroxide, facilitating polymer degradation.5 This corrosive environment accelerates the speed of degradation by 10× when compared with the rate observed during in vivo implantation.6
For our study, PU 55D tubing samples with 0.8-mm outside diameter and 60-µm wall thickness were used. While soaking, the samples were subjected to a constant pull force of 60 g. The hydrogen peroxide was exchanged three times per week. Samples were removed after one, three, five, eight, and 21 days of soak time. Degradation was observed only in the submerged regions. Examination with a scanning electron microscope (SEM) revealed surface roughness by day five and actual cracks (proof of ESC) by day eight.
|
Figure 4. This drawing shows an AFM tip with integrated heating element. |
Because the chemical change is largely a surface phenomenon, the use of nanoscale-thermomechanical analysis (nano-TMA) is well suited for measuring thermal property changes. The system used in this test, the nano-TA system from Anasys (Santa Barbara, CA)7, uses atomic-force microscopy (AFM) tips with an integrated heating element near the end of the cantilever. The deflection of the cantilever is monitored while the tip is heated. Initially, the deflection increases as a result of local thermal expansion of the sample and then decreases as the tip penetrates during the melting or softening of the material in contact with it.
|
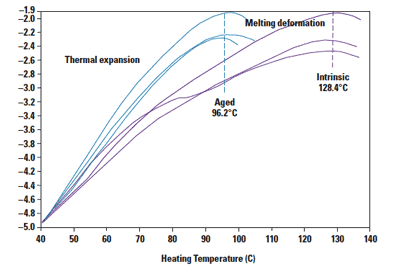
Figure 5. Nano-TA results show a clear decrease in the melting point on an aged sample. |
Samples submerged in hydrogen peroxide showed a clear decrease in the melting point from an intrinsic value of 128.4°C to 96.2°C. The lowered melting point is likely the result of chain scission and reduced molecular weight. Other segments of the same samples that were exposed to air retained the original melting point of 128.4°C.
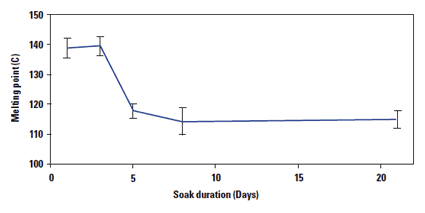
For a series of samples that were soaked for a variety of durations, the surface melting point was measured to decrease sharply at day five; the melting point then stabilized for soaks longer than five days.
When PU is subjected to a strong corrosive environment and high tensile force, ESC can occur quickly. Nano-TMA can identify a change in melting point in five days, three days earlier than cracks can be identified by SEM inspection.
In this study, very harsh conditions were used to demonstrate the degradation of PU 55D. Nevertheless, this polymer has shown to be reliable under typical-use conditions. R&D teams must demonstrate the reliability for each product made from the polymer. Employing a nano-thermal technique in addition to SEM can help identify degradation and cracks at an early stage.
|
Figure 6. The melting point of samples aged for various durations. |
|
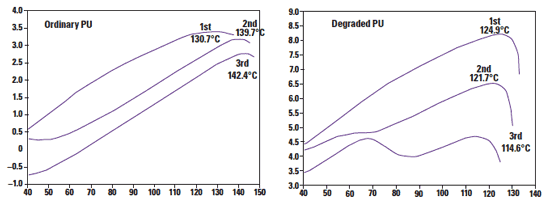
Figure 7. The image on the left shows repeated analysis showing phase separation of the aged polyurethane. The figure on the right illustrates how this effect is not observed with the sample, which is not subjected to aging in H2O2 (b). |
References
1. PA Gunatillake et al., “Designing Biostable Polyurethane Elastomers for Biomedical Implants,” Australian Journal of Chemistry 56 (2003): 545–557.
2. M Szycher and Chandra P Sharma, Blood Compatible Materials and Devices: Perspectives Towards the 21st Century, (Lancaster, PA: Technomic Publishing AG; 1991): 33–85.
3. EM Christenson, A Hiltner, JM Anderson, “Biodegradation Mechanisms of Polyurethane Elastomers,” Corrosion Engineering Science and Technology, 42(2), 312–323.
4. K Stokes, R McVenes, and JM Anderson, “Polyurethane Elastomer Biostability,” Journal of Biomaterials Applications 9 (1995): 321–354.
5. K Stokes, P Urbanski, J Upton, “The in vivo auto-oxidation of polyether polyurethane by metal ions,” Journal of Biomaterials Science, Polymer Edition, 1990; 1(3): 207–30.
6. EM Christenson, JM Anderson, A Hiltner, “Oxidative Mechanisms of Poly(carbonate urethane) and Poly(ether urethane) Biodegradation: In Vivo and In Vitro Correlations,” Journal of Biomedical Materials Research, 70A, (2004) 245–255.
7. K Kjoller, J Rose, and K Sahagian, “Transition Temperature Microscopy: Nanoscale Thermal Analysis for Micron- and Submicron-Scale Devices,” Laboratory Instrument News (2009).
Andy Hung has worked as a material scientist at UCLA, Second Sight Medical Products, and Advanced Bionics. Roshan Shetty is the co-founder of Anasys Instruments (Santa Barbara, CA) and Khoren Sahagian is an applications scientist at the firm.
About the Author(s)
You May Also Like

.png?width=300&auto=webp&quality=80&disable=upscale)
