Microfibers Extrusion: The Foundation for Biotextiles
High-definition microextrusion delivers unique fiber capabilities to medical device manufacturers.
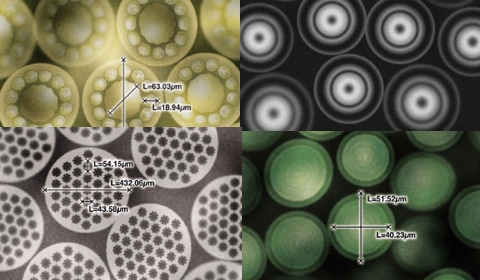
Medical microfibers and yarns are the critical building blocks of biotextiles. These specialized fibers are intricately knitted, woven, or braided into the biotextile components that are used in many of the implantable medical devices available today. The first medical fibers were developed decades ago for sutures, with fiber technology evolving to enable the biotextiles comprising the first cardiovascular grafts nearly five years ago.1 As fiber technology and the fabrication capabilities of these materials continues to be realized, new applications for the next generation of implantable medical devices, surgical fabrics, wound care, and tissue engineering applications are possible.
|
The medical-grade fibers and yarns used for implantable medical devices are produced through three primary techniques based on the extrusion of a polymer melt or solution:2
Melt Spinning. A polymer is heated to its melting point and is extruded through a spinneret to form continuous fiber strands.
Dry Spinning. Similar to melt spinning, however, the process starts with a polymer being dissolved in a volatile organic solvent to form a polymer solution. The solvent is later removed during a heated drying stage.
Wet Spinning. A polymer is dissolved in a suitable nonvolatile solvent and strands pass through a coagulation bath following extrusion from the spinneret to remove any solvent.
The economic, production, and scalability benefits of the melt-spinning process, without the use of solvents, has made this the preferred technique for medical fiber production today. Conventional melt-spin techniques are capable of producing fibers of single-polymer chemistry at high speeds and volumes. As melt-spin technology continues to evolve, resulting in enhanced fiber capabilities to include multipolymer systems for bicomponent or multicomponent fibers, the technology has become a commercially viable option. Dry spinning and wet spinning techniques can potentially be used to produce strands with multiple components, however work in this area remains in the research stages.
The next generation of implantable, textile-based medical devices requires the latest technologies for addressing the uniqueness of each application’s specific performance needs. When selecting a fiber supplier, medical textile manufacturers should consider partners who take a collaborative approach to product development to ensure project success. Another important consideration is to select a partner with a keen knowledge of polymer chemistry with the capability to fully characterize a polymer prior to extrusion. Fully characterizing the chemical composition and physical properties of the resulting fiber ensures the absence of polymer degradation byproducts. A well-aligned partner will also have an understanding of the medical device industry and the regulatory path, and will manufacture in a certified Class 10,000 cleanroom with compliant materials and validated processes. An aligned fiber partner will offer the most material knowledge while assuring their manufacturing process and related test methods remain validated and operate in a state-of-control.
One company that is focused on improving medical microfiber technology employs a unique melt-spin process known as ARmicron high-definition microextrusion (HDME). This technique uses nano- and micron-sized fibril components to form precise, unique structures within a fiber. The level of detail and definition using the HDME process enables intricate polymer domains within a fiber. This article focuses on the custom fiber and yarn manufacturing capabilities of the ARmicron HDME melt-spin process, which has application in a variety of implantable medical devices.
Fiber Customization
Nonresorbable | Resorbable |
Polyethylene Polypropylene Polyethylene Terephtalate (PET) Polyurethane (PU) Polyetheretherketone(PEEK) | Polylactic Acid (PLA) Polyglycolic Acid (PGA) Polylactide/Glycolide Copolymers (PLGA) Polycaprolactone (PCL) |
Table I. Examples of biocompatible polymers. |
Traditionally plastics and metals have been used as base materials for a wide variety of medical devices. However, biocompatible nonresorbable and resorbable fiber polymer choices are offering textile fabricators a wider range of material options for creating innovative, less invasive product constructions for a wide range of implantable medical devices (see Table I).
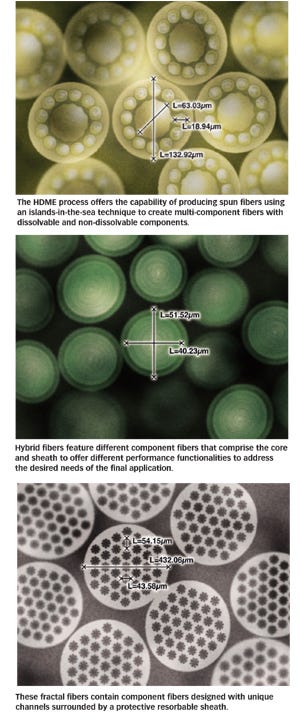
Creative use of these materials allows fiber manufacturers to produce fibers within fibers to take advantage of resorbable and nonresorbable properties simultaneously. The HDME process makes it possible to use up to four polymers during the manufacturing process to produce a wide variety of customized fibers. Another unique capability of the HDME process is the production of spun fibers with diameters of 300 nm using an islands-in-the-sea technique coupled with dissolvable and nondissolvable materials.3 This technique also enables the use of a small number of strong permanent fibers coupled with dissolvable fiber material to produce a lower profile final fabric.
The trend for small, minimally invasive devices intended for quick insertion and removal has a direct effect on the desired characteristics of a fiber. Fibers incorporated into the fabric portion of an implantable device must enhance the product’s conformability, be compressible, and be able to expand to offer flexibility and functionality in the finished device without restricting movement within the body.4 This demand is fueling a growing need for thinner and finer diameter fibers while maintaining tenacity. The HDME melt-spin process produces a unique islands-in-the-sea sheath-core fiber architecture as one way of addressing these needs.
In addition to the nondissolvable and dissolvable core-sheath fibers possible with the islands-in-the-sea approach, HDME provides the capability to produce hybrid fibers comprised of component fibers designed with different performance functionalities. For example, the inner fiber can offer the required strength needed, while the outer fibers possesses other properties such as a desired aesthetic, low melting properties for better bonding, inertness within the body to reduce inflammation, or barrier properties to provide a fluid barrier. The following section discusses the unique fiber capabilities of the HDME process in further detail.
|
Medical microfibers are knitted, woven, or braided as a critical component to form many medical implants. |
Fiber Characterization
All fiber manufacturers control the chemistry as well as the fiber’s physical properties, including denier, tenacity, elongation, and shrinkage to meet the specific performance requirements of an application. Producers of medical fibers require additional control to ensure potential polymer changes are minimized and well understood throughout the fiber manufacturing process via polymer characterization. Such controls include using physical, chemical, and analytical techniques on an ongoing basis. Various optical techniques such as scanning electron microscope are also useful for the determination of fiber uniformity, surface roughness, and identification of defects during the fiber development process. The optical techniques can also be implemented as tools for use after fiber development is complete if so desired.
Fiber Architecture
Conventional melt-spin processes typically produce fibers based on one polymer type, while fibers produced from the HDME process can be developed by coextruding multiple polymers to create specific polymer domains within the fiber. The process enables customized fiber constructions including monofilament, multifilament, and low denier per filament (DPF) yarns from a wide range of select biocompatible nonresorbable or bioresorbable polymers. Also unique to this process is the ability to create one-of-a-kind fiber architectures unachievable through other techniques. The HDME process leverages multicomponent extrusion capabilities to create desired fiber cross-section designs for increased functionality.
|
The HDME process can produce fibers that incorporate up to four differing polymers to build transition gradients. |
Using this approach, a fiber can feature up to four differing polymers of varying architectures including noncircular, noncentric, transition gradient, and sheath-core architectures. Hybrid fibers can be created using these techniques and by combining resorbable and nonresorbable fibers. The islands-in-the-sea image features multicomponent fibers with highly resolved internal domains using a modified sheath-core architecture approach for a surgical fabric to provide strength and support for cell growth. Over time, portions of the fibers will dissolve making the fabric less invasive while offering continued strength with the remaining inner nonresorbable fibers.
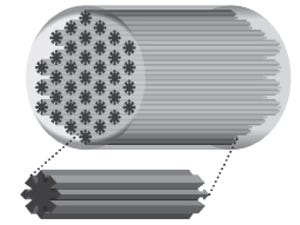
Fibers can also be developed with external submicron surface architectural definitions that may be beneficial for directional cellular ingrowth. The regeneration of some tissues, such as tendons, ligaments, nerves and muscles require cell alignment on one or more axes to optimize their functions.5 As seen in the fractal fibers image, the HDME process is capable of creating a continuous non-Euclidian external lateral fractal surface architecture simultaneous with internal spatially and gradiently resolved fiber domains for custom scaffold and fiber designs.
The HDME process enables the use of multiple polymers to produce a fiber’s internal structure combined with a protective sheath, while providing the desirable physical properties conducive for textile manufacturing. Because the typical weaving and braiding manufacturing processing can potentially cause the collapse of a fiber’s nanometer surface structures, the protective sheath preserves the internal architecture during the textile process and can be removed at a designated time during the process to maintain the fiber’s architectural integrity.
Applications for Today and the Future
Medical fibers have historically been used within the fabric components of implantable medical devices such as surgical fabrics, sutures, stents, and grafts for short- or long-term use. HDME technology is a custom product design alternative to traditional filament-fiber science. This process offers precise control of both external architectures and internal matrix morphologies for specialized and model applications to medical fibers with potential use in implants, medical textiles, and wound healing products.
|
An illustration of a fractal fiber core shows the aligned channels of the fractal surface, which creates grooves to enable cell alignment and topographical control of cell orientation. |
As it relates to tissue engineering, 3-D micro-nanofabrication of the HDME fiber design helps transition tissue scaffold designs away from traditional Euclidian geometries to specialized fractal designs. HDME precisely and simultaneously produces both external non-Euclidean (i.e., lateral fractal nanopattern) and internal gradient transition cross-section, which can support the assembly and “trophic-doping” for improved scaffold features.
Product developers may opt to load custom-designed gradient microfiber architectures for specialized tissue engineering, scaffold design, or wound healing with cross-section transition domains that promote controlled release, spatially resolved growth agent incorporation, and managed multicomponent biopolymer matrix biodegradation. A future capability of the technology may offer product developers the option to combine controlled release with custom architecture.
Conclusion
The development of the next generation of medical devices will be fueled by advancements in component materials to deliver the enabling technologies to turn product concepts into realities. New fiber material options and capabilities available through high-definition microextrusion melt-spin technology create customized fiber constructions including monofilament, multifilament, and DPF yarns select biocompatible nonresorbable or bioresorbable polymers to create unique and enabling fiber architectures. The use of HDME techniques coupled with collaborative custom fiber development, clean manufacturing, polymer/product characterization, and ingenuity can make new applications in medical implants, wound care, and drug delivery devices a reality.
References
1. JM Koslosky, “Biomedical Textiles Stay in Step with the Beat of Cardiovascular Designs.” Medical Design Technology (2011).
2. K Tuzlakoglu, L Reis, “Biodegradable Polymeric Fiber Structures in Tissue Engineering.” Tissue Engineering: Part B 15 (2009): 17-27.
3. Hills Inc., “Production of Sub-micron Fibers in Non-Woven Fabrics,” Barrier Fabrics of Spunbound Specialty Fibers for Medical Applications (West Melbourne, FL, 2012).
4. Secant Medical, “What are Biomedical Textiles?” (April 2012).
5. Q Lu, A Simionescu, and N Vyavahare, “Novel Capillary Channel Scaffolds for Guided Tissue Engineering,” Actabio Materialia (2005): 607-614.
| Greg Toney is manufacturing manager for medical fibers at Adhesives Research Inc. (Glen Rock, PA). He focuses on the organization and production processes for permanent and biosorbable fiber structures. He holds a BS in chemistry from Wofford College and a MS in chemistry from Furman University. He has 36 years experience in material science, polymer processing, and engineering in extrusion, coating, textil,e and nonwoven processing, and polymer additives. Reach him at [email protected]. |
| Ming Wei, PhD, is product development chemist at Adhesives Research. Prior to joining the firm she was a research professor in the plastics engineering department at university of Massachusetts Lowell (UML). She obtained her doctorate degree in plastics engineering from UML and has been working in the field of polymer science and engineering since 1996. Reach Wei at [email protected]. |
| Bob Wigdorski is senior research & development scientist at Adhesives Research. He is responsible for the development of adhesive technologies for industrial and electronics markets, and provides technical support for the covert marker authentication technologies and medical fibers portion of the firm’s product portfolio. He has 42 years experience in the adhesives and coatings industry and graduated from the the State University of New York at Buffalo. Contact him at [email protected]. |
About the Author(s)
You May Also Like






.png?width=300&auto=webp&quality=80&disable=upscale)
