October 1, 2014
A person's heart beats roughly 100,000 times in one day and more than 2.5 billion times in a typical life span, according to PBS. Imagine the challenge of developing an artificial heart that can match that durability.
One of the difficulties was finding a material that is up for the job. Total artificial heart maker SynCardia Systems Inc. (Tucson, AZ) found that segmented polyurethane was ideal for the task. After testing the material against an array of different materials, the company found that nothing could beat segmented polyurethane when it came to stretching, strength, and shape memory. In particular, the biocompatible material has remarkable flex life properties, making it well suited for use in artificial hearts.
A few years ago, however, the company's supply of the material was threatened. SynCardia decided to look around for alternatives. "We tried working with half a dozen different companies that specialize in materials," says Isaacs. They couldn't find a material that could beat it.
|
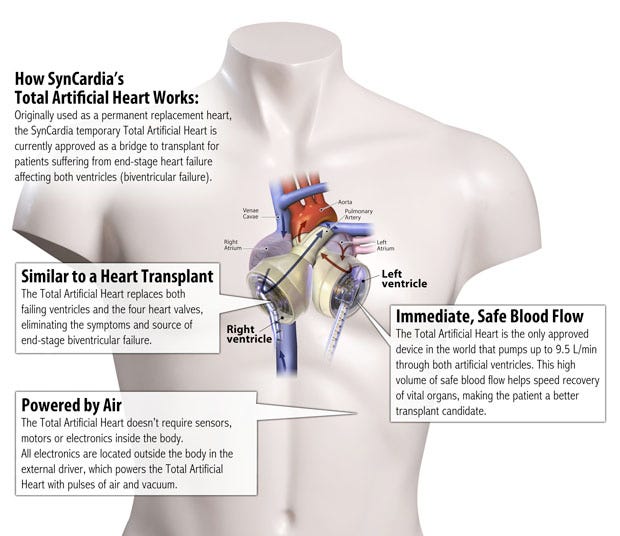
SynCardia's artificial heart |
SynCardia decided to buy the material's formula, the equipment to make it, and everything else needed to make it in house. SynCardia is now the only company to manufacture the material. "We now have a whole team that does nothing but make the material for the SynCardia Total Artificial Heart," says Don Isaacs, the company's vice president of communications.
The company's artificial heart has always been made out of the material since the predecessors of the modern SynCardia temporary total artificial heart were first implanted into human patients 30 years ago. And now that it makes it in house, the company can retain control over its formulation.
Segmented polyurethane, however, has a storied history in the medtech history. A research paper from 1967 was published in Science hailing the material as a promising new elastomer for biomedical applications.
The material, first developed to make elastic thread, was used in the late 1960s for molded prostheses. But the material's suitability for cardiac applications was hinted at in the Science abstract, too. "Performance of this material when used for components of a heart-assist system warrants a thorough investigation of its effectiveness in a variety of biomedical devices," reads the last sentence in the Science abstract.
The SynCardia's heart is the first and only total artificial heart to be approved by FDA as well as regulatory bodies in Europe and Canada.
Segmented polyurethane helps the device's diaphragm to have a reliability rate greater than 99.5%. "The device is elegant in design. All of the sophistication is in the design itself so there is no need for electronics inside the body. All electronics are safely located outside the body in the pneumatic drivers that provide preciously calibrated pulses of air that make the total artificial heart beat.
"The first total artificial heart with our technology was in 1982 and he didn't die from the heart. He lived 112 days after it was implanted. Our device has the highest bridge-to transplant rate of any approved artificial heart or ventricular assist device and t it is the only device that eliminates the source of heart failure."
From the first artificial heart implant in a human patient in 1969 through September 5, 2014, there have been a total of 1413 artificial hearts implanted. The SynCardia Heart and its predecessor designs were implanted a total of 1352 times. SynCardia says it is responsible for 96% of artificial hearts that have been implanted into humans.
Refresh your medical device industry knowledge at MD&M Chicago, October 15-16, 2014, and MD&M Minneapolis, October 29-30, 2014. |
Brian Buntz is the editor-in-chief of MPMN and Qmed. Follow him on Twitter at @brian_buntz.
Like what you're reading? Subscribe to our daily e-newsletter.
About the Author(s)
You May Also Like