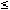A Practical Guide to ISO 10993-12: Sample Preparation and Reference Materials
Medical Device & Diagnostic Industry Magazine MDDI Article Index An MD&DI December 1998 Column ISO 10993
December 1, 1998
ISO 10993
Critical to all types of biocompatibility studies, the methods for preparing device materials for testing are covered in this standard. Note: this is the continuation of an ongoing series of articles on ISO 10993. Last month's installment covered hemocompatibility.
Developed by the International Organization for Standardization (ISO), the set of standards known as ISO 10993 address the important issue of proving the safety of medical devices by identifying various types of biocompatibility tests. Because the method used for preparing device materials for testing is critical to each study, sample preparation and reference materials are covered in ISO 10993-12. The standard describes the types of test samples, suitable extraction vehicles and conditions, and appropriate reference materials to be used as controls. This article reviews the standard's requirements and recommendations in each of these areas.
TEST MATERIAL SELECTION
To ensure patient safety, the biological evaluation of medical devices must go beyond the testing of constituent materials. The goal of such testing programs is not only to confirm the safety of individual device materials but also to confirm that manufacturing steps will not compromise the biocompatibility of the device. Processing aids, mold release agents, lubricants, and other additives, as well as cleaning agents and sterilants, can have adverse effects if they contact the body; therefore, the samples used for testing must be selected to take these factors into account.
The standard recommends testing medical devices in their final product form and condition whenever possible, except for selected tests (e.g., implantation) that may require that individual materials be evaluated separately. If finished devices will not be available for testing, the evaluation of representative subcomponents of the device is acceptable in some cases. As a last option, representative samples of the formulated materials that have been preconditioned by the same processing steps as the final product should be tested. If the device is too large or cannot be tested as a whole for some other reason, each individual material having the potential of coming in contact with body tissues should be represented in the same proportion in the test sample as it is in the final product. In all cases, the standard requires that the samples be handled in such a manner as to avoid contamination.
PREPARATION OF EXTRACTS OF TEST MATERIALS
Medical device materials present a unique challenge to toxicologists, whose experiments usually involve chemical substances that can be delivered to a biological test system such as a cell culture in a measurable dose. Because devices are made of plastics, metals, and other solid materials, defining specific doses of the substances of interest is generally not possible. For most tests, the preparation of fluid extracts of the device materials is the most appropriate technique to provide test samples for determining the biological reactivity of possible chemical leachables (Figure 1).
 Figure 1. A 50-ml extraction vial containing 60 cm2 of test article covered with 20 ml of minimal essential media for cytotoxicity testing.
Figure 1. A 50-ml extraction vial containing 60 cm2 of test article covered with 20 ml of minimal essential media for cytotoxicity testing.
According to the standard, the fluid used for extraction and the extraction conditions should be appropriate to the final device and its end use. It is critical that the various extraction media selected for testing represent the environments in which the final product will be used. Physiological saline and vegetable oil are usually sufficient to provide polar and nonpolar environments. The saline extracts water-soluble chemicals, while vegetable oil extracts lipid-soluble chemicals; both types of chemicals can be extracted by various fluids in the body. Depending on the nature and use of the device or requirements of a specific test method, other fluids also may be used as extractants provided that the effects of the fluids are known.
Extraction should be carried out at temperatures that are high enough to maximize the amount of extractable substances as well as to simulate the highest temperatures the device may be exposed to before or during use. However, extraction conditions should not cause deformation or degradation of the test or control articles. A number of specific acceptable extraction conditions are outlined in the standard, including 37ºC for 24 hours, 37ºC for 72 hours, 50ºC for 72 hours, 70ºC for 24 hours, and 121ºC for 1 hour.
For most test materials, extractions are performed under static conditions. However, agitation may be deemed appropriate as an effort to more closely mimic an end use or to ensure that the extraction media come in contact with all relevant device components. In any case, when agitation is considered appropriate, the method used should be documented.
Thickness | Extraction ratioa | Examples of Material |
|---|---|---|
| 6 cm2/ml | Metal, synthetic polymer, ceramic, composite film, sheet, and tubing walls |
>0.5 | 3 cm2/ml | Metal, synthetic polymer, ceramic, composite tubing walls, slab, molded items |
| 3 cm2/ml | Natural elastomer |
>1.0 | 1.25 cm2/ml | Natural elastomer |
Irregular | 0.1–0.2 g/ml, | Pellets |
a Expressed as the ratio of the surface area or mass of the test sample to the volume of extractant used. |
Table I. Suitable extraction ratios for test materials of various thicknesses. (Adapted from ISO 10993-12.)
The amount of test material used in the extraction process is usually expressed as a ratio of sample surface area to extractant volume or sample mass to extractant volume. Generally speaking, the surface area ratio should be used whenever possible, with a mass-to-volume ratio used only for the testing of irregularly shaped devices or representative device components. The extraction ratios specified in the standard are outlined in Table I. However, in some rare cases it may be necessary to deviate from these ratios and doing so is considered acceptable as long as the ratio of test material to extractant simulates or exaggerates the conditions that will be encountered during clinical use and the ratios used are documented in the test results.
The standard also notes that there are no standardized methods available for testing absorbents and hydrocolloids and suggests the following protocol. Using 2 g of the material as a test sample, determine the absorption capacity of the sample—that is, the amount of extractant absorbed by the material. The extract volume should then be 20 ml more than the sample's absorption capacity.
REFERENCE MATERIALS
In nearly every biocompatibility test, reference materials are used to serve as experimental controls. Negative controls, in the form of blanks, are used in most biological evaluations where test article extracts are prepared. The use of these blanks provides the basis for a comparison of the effects of the test material extract with a validated negative test result.
A number of materials have been used extensively in biological testing as negative or positive controls. High-density polyethylene, obtained from the U.S. Pharmacopeia, is a standard negative control. The nonreactive plastic can be implanted into living tissue and the results compared with those for a test material that has been similarly implanted. Likewise, a polyvinyl chloride formulation containing organotin additives serves well as a positive control.
CONCLUSION
ISO 10993-12: "Sample Preparation and Reference Materials" clearly indicates that it is preferable to evaluate medical devices in their final product form. The reasoning is simple—the biological testing must incorporate everything involved in making the device. Obviously, the constituent materials must be safe for patient contact; equally important to device biocompatibility are the processes and materials used during manufacturing. For most devices, the use of fluid extracts of the test materials prepared in a fashion to mimic or exaggerate the expected clinical conditions is the most appropriate technique for determining the potential effects of chemical leachables. Extraction fluid selection, extraction conditions, and material-to-extractant ratios are all outlined in the standard. The selection and use of appropriate experimental controls also is important in evaluating device materials for safety and is also covered in ISO 10993-12.
Timothy Jansen is acting manager of toxicology, California division, and Richard F. Wallin, DVM, PhD, is president of NAMSA (Northwood, OH).
Copyright ©1998 Medical Device & Diagnostic Industry
About the Author(s)
You May Also Like