Stent- and Catheter-Delivery Tubing: What Is Your Tolerance Level?
March 12, 2015
To produce tubing for stent- and catheter balloon-delivery systems, manufacturers employ a range of dimensional, tolerance, and quality strategies
Steve Maxson
|
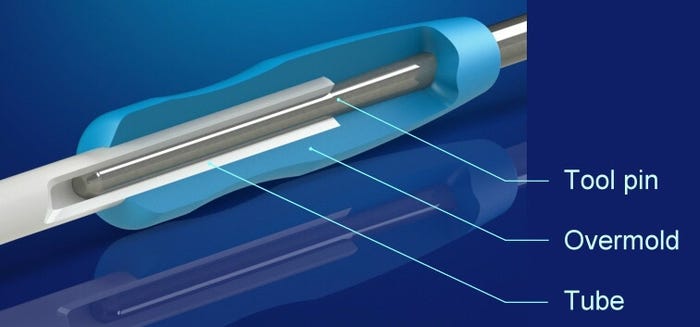
Tubing dimensional variations can negatively impact such secondary operations as overmolding. |
Medical extrusion providers are increasingly expected to produce complex, close-tolerance tubing that meets the functional design requirements of minimally invasive stent- and catheter balloon-delivery systems. This task is rendered all the more challenging because such devices must incorporate tubing with different durometers. For example, the tubing used at the proximal end of a catheter is made from stiffer materials than that used at the distal end.
To produce such tubing, manufacturers must employ a range of dimensional, tolerance, and quality strategies. At the same time, they must understand the impact that dimensional variations can have on such high-quality secondary operations as overmolding and tip forming.
Dimensional Strategies for Extruded Tubing
In such medical devices as catheters, single-lumen tubing includes such specifications for the final tubing dimensions as outside diameter (OD), internal diameter (ID), wall thickness, concentricity, and cut length. Delivery systems often require that the tubing ID meet tight tolerance requirements to enable a guidewire or other device to pass through the lumen. They also require that wall thicknesses meet tight tolerance and concentricity levels in order to withstand burst pressures. In contrast, the key dimensional attributes associated with such postextrusion processing operations as overmolding and tip forming are the OD and ID.
From the outset, manufacturers should be clear about the tolerance levels they expect when controlling critical tubing dimensions and how these levels are to be interpreted during a lot or extrusion run. For example, during a given extrusion run, the OD, ID, and wall thickness dimensions may fall within the literal upper and lower tolerance limits, or must they may fall within a statistical process control (SPC). These scenarios are very different from each other.
The most widely used statistical measure of process capability in medical extrusion processes is the process capability index (Cpk), which measures how close a process runs within dimensional upper and lower tolerance limits. The typical Cpk value used for a controlled extrusion process is 1.33, which means that 99.994% of the tubing is within dimensional specification.
Depending on the resin, quality of the extrusion equipment, and how tight the tolerance levels are, a cpk of 1.33 is not always easy to achieve. In order to validate an extrusion process, a balance must be struck between how tight the tolerance levels are and the required process capability. Thus, the processing window should compensate for a certain amount of dimensional and material lot-to-lot variation.
In closed-loop controlled extrusion processes, an OD gauge can be used to measure and control the OD while an ultrasonic wall gauge can be used to measure and control the wall thickness. But because such processes calculate the ID based on the OD and wall-thickness readings, these measurement technologies deliver inaccurate online readings because they cannot exactly pair wall readings with OD readings. Consequently, the ID is usually measured offline using a pin gauge squeezed into the tubing ID, although a more reliable and accurate approach relies on the use of a noncontact vision system that does not deform the tubing.
Overmolding and Tip-Forming Operations
The process of overmolding a luer or hub to one end of a catheter tube compresses the tube's wall thickness between the ID and OD. This compression results from the use of a mandrel or core pin on the ID to support the tubing while the mold closes around the OD. The compression of the tubing is strongly influenced by the hardness of the material--a factor that impacts the overmolding process. The compression levels of such materials as low-durometer Pebax or polyurethane differ from those of stiffer materials such as nylon 12 and PEEK.
When a tight tubing ID tolerance is required to enable a guidewire to pass through the lumen and to ensure that the wall thickness can meet burst-strength requirements, the OD serves as a reference dimension that is not held to a tight tolerance or to a Cpk. However, this procedure can lead to an interface between the tubing OD and the mold cavity that is too close to the upper or lower tolerance limit, resulting in an interface that is either too loose or too tight. If the interface is too loose, the injection-molded material will escape from the cavity between the extrusion OD and the steel cavity, creating flash. Alternatively, it can cause the tube to be pushed off of the mandrel. If the OD is too large, the cavity will pinch into the wall. While this may result merely in a cosmetic issue, it can also potentially compromise the tubing's burst strength.
|
Soft atraumatic tip on the distal end of the catheter. |
The bonding/tipping die cavity is designed to accept maximum-diameter tubes based on their nominal OD dimensions and upper tolerance levels. OD dimensional variations beyond these levels can compromise the quality of the soft atraumatic tip on the distal end of the catheter. For example, a wide OD tolerance range can generate a lip, or stepped transition, from the tip area down to the extruded catheter shaft OD, potentially necessitating a device redesign or a tightened OD tolerance level. Higher-durometer (stiff) tubes can also be affected by ovality. This phenomenon causes the tube to act as if its OD is oversized, preventing it from conforming to the tipping die because one axis exceeds the OD tolerance specification.
While a design change can include a tapered transition to absorb the tolerance range, special care must be taken during setup to ensure a consistent starting point for the tubes to minimize the impact of other tip-forming parameters. For best results, the OD tolerances levels should be held to ±2% for rigid tubes and ±3% for flexible tubes.
Conclusion
Because stent or catheter tubing tolerances may not coincide with the extrusion, molding, and tipping manufacturing requirements, there is considerable value in working with a polymer solutions provider that understands the device design and can perform extrusion and such secondary operations as overmolding and tip forming. While optimizing device performance, this integrated approach enables the manufacturer to maintain high quality levels, manage costs, and reduce delivery times.
Steve Maxson is technical sales manager at Raumedic Inc. (Leesburg, VA). Reach him at [email protected].
About the Author(s)
You May Also Like