Right Sizing Implantable Pressure Sensors
Sharing industry best practices in the development of implantable sensor devices is critical to define a pressure-tested approach for the future.
September 5, 2023
By Dave Fromm, Promex, and Kevin Berg, Injectsense
More uses for implantable sensors are being discovered every day. One such capability is pressure detection, including the detection of intra-ocular pressure, helpful for treating glaucoma.
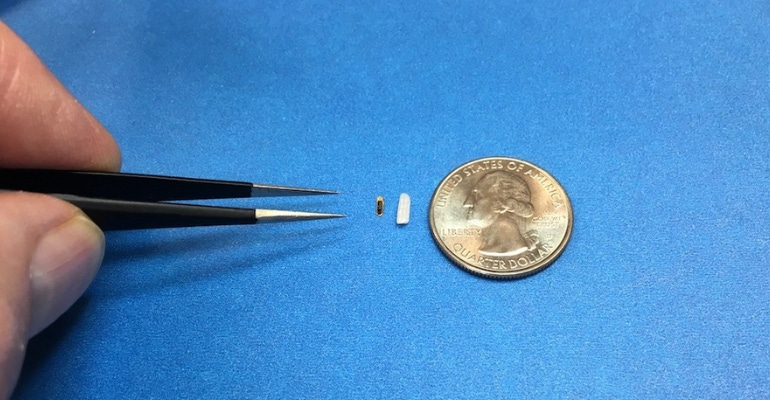
Advances in integrated microelectronics have enabled a revolution in ever smaller and more powerful sensors. The implanted sensors collect direct dynamic pressure and transmit high-fidelity data from inside the body to the outside world, requiring less power as innovation advances their functionality and energy efficiency. Smaller sensors can more easily be injected and are less invasive for the long-term comfort of the patient. For intra-ocular pressure detection, the device is smaller than a grain of rice.
How can an OEM balance the therapeutic opportunities along with the challenges of miniaturization when bringing its first- and next-generation implantable sensor devices to market?
The answer is complex. It involves not only right-sizing the device, but also focusing on the correct development strategy across multiple domains, from electrical to mechanical, assembly, manufacturing, and handling — all including scalability and repeatability — to ensure that high-quality standards are maintained for each assembled device.
From our experience working side-by-side to produce an advanced, miniaturized intra-ocular pressure sensing device, here are some tips and techniques we’re taking along the way as we progress toward commercialization and scalable manufacturing.
The new frontier
OEMs and their production partners are breaking new ground in sensors due to advances in MEMS technology and complexity, a higher density of features in semiconductor materials within the device mounts, and innovation in advanced packaging and assembly. Additional advances in microelectronics and ultra-low power sensing and digitization enable extreme, low-energy data storage that permits the detection of specific events in the body. These, in turn, provide clinically actionable information that may be related to cardiac cycles, changes in body position, body activity, temperature, respiration, and the like.
Just 10 years ago, producing a device like this would not have been possible. Today, the market is continually pushing to scale the sizes of devices down by many several factors as technological advances allow more functions to be placed within less space. Additionally, the design and development of the devices must allow production at scale to meet increasing market demand.
These challenges call for specialized tools and a customized approach to manufacturing. While the standardization of manufacturing may be more commonplace in another decade or so, there currently are no standards for manufacturing and assembly of implantable sensor devices. Consequently, each product or device has its own set of components and necessary tools and processes for production. The geometries are unique for each product, with specific requirements. Today’s approach to manufacturing these devices is mix and match, yet there are some best practices emerging to bring these complex products to commercialization.
A smart start
Injectsense’s implantable intra-ocular pressure sensor platform consists of:
An ultra-sensitive, ultra-precise pressure transducer
Inductive coupling to power the device and to facilitate bidirectional data communication
An ASIC chip for onboard digitization and storage of data and management of power and wireless communication
Its materials are assembled into the completed device. Given the delicate components, miniaturized geometries, and the development expense of creating such a small yet powerful implantable device for medical use, it is important to anticipate, plan for, and manage risk at every stage.
After the prototype development phase, we began partnering together. An outside partner such as Promex adds a second set of eyes on the device and can help brainstorm an approach and workflow to move from prototype to production. Such a plan can avoid potential mechanical damages (dents, scratches) or surface contamination from processing residue. Together, we identified potential complications and associated risks throughout the proposed assembly process.
Documentation at this stage is an absolute requirement for medical devices under design control. It provides visibility to all partners involved in the production process and establishes a clear roadmap and communication between parties, one of the greatest factors in the success of a project. Documentation accuracy and maturity should track alongside the product itself, with specificity and scope increasing as the production phase approaches. While ‘perfection’ is the end-goal, establishing a broad framework and filling it in as learning increases is a recommended best practice, and must be rigorously managed under both parties’ harmonized Quality Management Systems.
Moving into production
There are multiple steps involved in device assembly, a proprietary process designed to preserve performance even when the device is exposed to media including blood and tissue. Production is a key factor in achieving the low drift and high stability needed for accurate measurement, yet manufacturing must be straight-forward and scalable. In the case of our device, we have a drift of less than 0.01mm Hg per month.
With outside input and a plan for production, we updated our design of the device parts and fixtures, including the containers used to transport the device in its various stages into and off of the production line, to mitigate misuse during assembly. Process controls were also put in place if changes turned out not to be feasible.
One example of a change we made was switching out a material that was not compatible with the temperatures used during the proposed assembly process. Additionally, some originally designed tolerances and dimensions were not resulting in the desired high quality and yield, so the stack-up and associated documentation were updated.
Assembly and manufacturing
During the planning stage, assembly into the carrier was identified as the step that posed the greatest risk. During this stage the production team had to ensure adhesives used in the process ended up where they were supposed to be, as the potential for the adhesives to end up in the wrong place and on the wrong part were particularly high.
When using adhesives, a team must consider several important factors such as viscosity, dispense tolerance (volume and position), surface interactions with various components and materials (usually best done through experimentation), and the inherent considerations of strength, operating condition range, and cure schedule.
Mechanical fitting to tolerance is another important area of consideration in the carrier assembly stage as, at small sizes, the tolerance stack-up for fabrication tolerances and location can become significant issues. Early on Injectsense used comprehensive modeling to predict and simulate tolerances, including Solidworks models and other design tools. The maximum allowable variation that preserves design intent is the sum of fabrication and assembly tolerances. Within a small assembly, capability limits of even high-precision fabrication techniques often use most of this budget, leaving little room for assembly variation.
Finally, it’s important to understand how coefficient of thermal expansion (CTE) differences impact assembly for various materials within a small device. These forces can be very strong over a tiny area. Many parts break and/or warp during assembly when CTE is not considered. Prior to manufacturing, Injectsense used very accurate geometric descriptions and other data to conduct extensive Failure Mode and Effects Analysis (FMEA) that helped us to understand the properties and what was required to avoid failure modes and mechanisms.
For assembly and manufacturing, other important considerations include:
Ultra-miniature form factors (in this case <2 mm3) require precision and die-to-die bonding and assembly with high repeatability across process tolerances. Effective parts handling during this stage will limit damage and a well-controlled cleaning process of all surfaces during production will help to avoid contamination that can bring negative repercussions on downstream assembly and assembly reliability.
Hermeticity, referring to the ability to keep moisture and gases out of an enclosure comprised of inorganic materials, is required for the decoupling of sensor electronics from the media. While many devices use a titanium can, Injectsense’s sensor is the entire hermetically sealed package. Hermeticity enables electrical and mechanical decoupling in order to maintain performance in media under a wide range of body conditions and temperatures. Media may include cerebrospinal fluid, vitreous, blood, and tissue. For hermeticity with long-term isolation from media (preventing fine and gross leaks), only metal bonding was used. In our process, we applied an additional external coating for biostability. Using a can or polymer will ultimately cause time-delay failure. Polymer anywhere induces more drift and is unstable in volume.
For the integration of a solid-state rechargeable battery (thin film), it is important to consider that typically 80% of a standard sensor package volume is dedicated to energy storage. For our device, we were able to use far less volume due to the increased power density in our thin film battery cell. The microbattery has the same footprint as other components (another layer in the stack) and still matches target performance. For this step, we take the same assembly approach in die-to-die bonding throughout the stack but need to keep the microbattery in a chemically inert environment.
Manufacturing conclusions
To produce integratable components while eliminating assembly surprises, the 3D integration modular approach and commonality of process modules enables improved reliability and management of process tolerances.
Interfaces can be a point of failure — interfacing with media and between components. With precision, the assemble, die coat, and manage vias (through wafer) throughout the entire stack with predictable connectivity, controlled parasitics, and hermetic sealing from media, can reduce risk of failure.
Use of Product Lifecycle Management (PLM) software will provide more effective management of the design transfer to manufacturing, controllably tracking revision changes of parts, assemblies, assembly/test processes, and their supporting documentation.
FMEA and Design-for-Test, Design-For-Manufacturing, and Design-For-Assembly (DFx): Anticipating manufacturing and test challenges reduces design trade-off uncertainties and identifies tolerances and needed test structures to support complete observability and controllability at the design level (either for the assembly or the manufacturing process).
Supply chain management
During the recent supply chain shortages and challenges that the medtech community has faced, we all instituted the best practice of proactively managing supply chain strategy as early as possible in the project plan to minimize production disruptions.
In our case, the raw components were sourced by Injectsense, and the materials used to build the assemblies (adhesives, solders, films, etc.) were sourced by Promex. Ensuring adequate supply from reliable sources significantly increased the reliability of the project. Long lead times and short expiration dates remain a challenge and, more than ever, material availability and supply chain continuity are key factors in driving key design decisions.
Material selection is also important. For medical device OEMs, material needs are varied and often critical for biocompatibility or to ensure proper quality systems/certificates of conformance from upstream suppliers.
Machines to build implantable devices at high volume also have very long lead times — six months to a year is common. This is one of the larger constraints. Having a right-sized, at-the-ready production line for your unique implantable device will allow for scalability.
Packaging, handling, and shipping
Reducing the number of partners involved in the assembly and manufacturing of an implantable device is key. The ultimate partner will have as many capabilities under one roof as possible, which will limit the movement of the device at various stages on and off the production line, from one location to the next, during production. This vertical integration/turnkey approach of assembly capability greatly shortens time to market by simplifying logistics, reducing handoff risks, and streamlining change implementation, as only a single set of process documentation and manufacturing workflow is impacted.
Packaging and handling, just like assembly, can be tricky to manage when unfinished assemblies are shipped to various locations for various steps. At Injectsense, we designed shipping fixtures to mitigate damage risks, and Promex offered additional insights into how to reduce risk to components during shipping, handling, and the ingress/egress of parts for each assembly step, such as ways to reduce static electricity to keep parts stable and fixture geometries to make parts easier to grasp.
We experimented with different materials and geometries for the packaging fixtures, making small changes, like having components sit inside a uniquely designed cavity, to big changes, like designing the fixture so the part doesn’t even need to be removed from its package during an assembly step.
Reducing the number of manual touchpoints at various stages and designing the containers around production steps should be implemented before production begins. This will reduce breakage and production time and increase yield.
As there is not yet a standardized process for producing implantable sensor devices, sharing industry best practices is critical to define a pressure-tested approach for the future. Despite the complexities and variances inherent in every project, yielding the right results is possible when using the right team and partner. By planning out every step for bringing our intra-ocular pressure sensor for the eye to the market, our considerations and experimentations ultimately led to the right results: achieving a significant increase in production yield of the device, to 98%.
About the authors:
Dave Fromm is COO and vice president of engineering at Promex, the market leader in microelectronic component assembly, process design, and packaging for the medtech and biotechnology markets. Promex has the broadest set of capabilities under one roof: two full SMT lines; post-wafer processing technologies, including wafer dicing, grinding, die attach, and flip-chip; wire bonding; encapsulation; and more. With cutting-edge equipment, extensive material properties knowledge, and meticulous supply chain management, Promex partners with OEMs at all stages, from early and small prototypes to R&D development and high-volume production.
Kevin Berg is COO at Injectsense, which, at the intersection of advanced semiconductor technology and medical systems, develops implant-based digital health systems to provide medical staff with continuous long-term data for improved therapy management.
You May Also Like

.png?width=300&auto=webp&quality=80&disable=upscale)
