Micromolding the Future of Medical Technology
May 15, 2015
The company MTD Micro Molding specializes in molding tiny parts for the medtech industry.
Brian Buntz
|
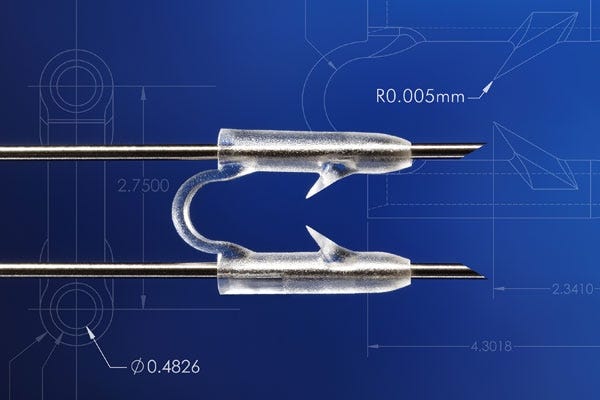
Above is a component molded by MTD Micromolding. |
In 2009, Bloomberg named miniaturized medical equipment as one of the most important breakthroughs of the next 10 years. Advances in everything from electronics to micromolding have helped make it possible, leading to such eye-opening announcements such as a surgical robot with such precision that it can suture a grape together.
This trend though, if it can be called that, has been obvious for decades. The company MTD Micro Molding (Charlton, MA) has long built its business on making miniature parts. Founded in 1972, the firm cut its teeth making miniature connector molds for the electronics applications.
Originally known as Miniature Tool & Die, the company was founded in a brick building in Worcester, MA, originally making the miniature tools and dies that were its namesake.
After the move to the newly built facility in Charlton, it gradually started doing more micromolding in its facility until it reached the point that 80% of its business came from that. In 2010, it changed its name to MTD Micro Molding to reflect the shift. (The company is exhibiting at MD&M East in New York City, June 9-11, 2015 at Booth #875.)
Now, 100% of its business comes from the medical device industry.
"Medical micromolding is what we do. And all we do," says the company's project manager Lindsay Mann.
|
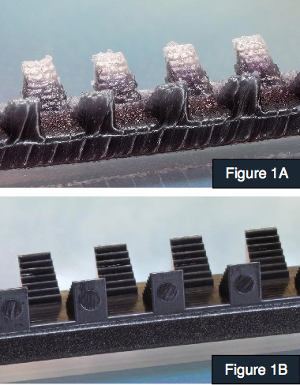
Figure 1A shows a 3-D printed component. Figure 1B shows the same component molded by MTD Micro Molding. |
One fifth of the company's business comes from what it terms rescue projects. "A company could have gone to another molder with their design and ask them: "Can you make this?" "They'll say, 'yes,'" but then they are unable to make the part to the print and they come to us," Mann says.
Molding tiny parts--especially from exotic materials such as bioabsorbable resins--is not as easy as it may seem. "Some companies may assume that a thermoplastic shares the same molding properties as a bioabsorbable so it is treated the same way," Mann says. "Across the board with materials, the rules are different when you mold it so small. We have had to make our own material database that lets us predict how a specific material will flow in an application because there isn't much external data out there on it."
Similarly, another pitfall some molders fall for is assuming macromolding rules apply to micro molding--one that is compounded by using bioresorbable resins. "The rules are different when you are micromolding; the source of the problem is failing to understand that small, miniature volumes of any material behave differently than larger, normal volumes. We have had to rely on our own research and knowledge to master it," Mann says.
"For molding companies that don't have a lot of experience making small parts, the geometry of a component might look simple or easy to complete against the drawing. But when it comes time to create the mold for, say, bioresorbable staples, it is critical that the finished parts meet all drawing specifications," Mann explains.
Because bioresorbable staples are designed to stay in the body for six to nine months, it is crucial that the parts experience minimal and consistent IV (inherent viscosity) loss during the molding process. "And this can be very hard to do because the critical molding parameters like temperatures and pressures, involved in processing these materials can severely degrade the material, which will directly affect the absorption rate of the product in the body," Mann says.
Learn more about molding at MD&M East in New York City, June 9-11, 2015 and visit MTD Micro Molding at Booth #875. |
Brian Buntz is the editor-in-chief of MPMN and Qmed. Follow him on Twitter at @brian_buntz.
Like what you're reading? Subscribe to our daily e-newsletter.
About the Author(s)
You May Also Like

.png?width=300&auto=webp&quality=80&disable=upscale)
