Medical Packaging Professionals Continue to Challenge Redundant Stability Tests
Wicked Stability team members reconvened at thePACKout to discuss new workstreams and next steps for testing and proving the project’s hypothesis.
Following the success of last year’s inaugural event, KiiP’s Wicked Stability team members returned to thePACKout conference in May to share their progress and update attendees on new workstreams aimed at gathering data to support the project’s hypothesis.
For those just joining the conversation, KiiP’s Wicked Stability team is on a quest to eliminate repetitive stability testing activities in favor of a more scientifically rigorous method for predicting and validating material stability. But to do so, it must first prove that materials of construction (MOC) with thermal oxidation as their primary mechanism of degradation are protected against reduction in performance as long as antioxidant is present in those materials.
Revisiting their wicked ways.
The concept of Wicked Stability and its goals were first introduced to the industry last year during a session titled “Wicked Stability: Re-imaging Stability.” This year, Rod Patch, Senior Director, Package Engineering, Johnson & Johnson Vision, opened the session, “Wicked Stability: Project Progress and Workstream Updates,” with a recap of the Wicked Problem, reiterating the project’s driving force: Properly executed stability studies do not fail.
“We believe materials of construction for sterile barrier systems have been demonstrated as stable with redundancy across the industry for repetitively evaluating those materials, and that’s wasteful,” he said. “Maybe we can use our resources for better applications and innovation and better time use than constantly proving well-known materials are stable.”
According to Patch and the Wicked Stability team, measurement of degradation factors associated with temperature through oxidative induction time (OIT) might be a better approach to validating material stability.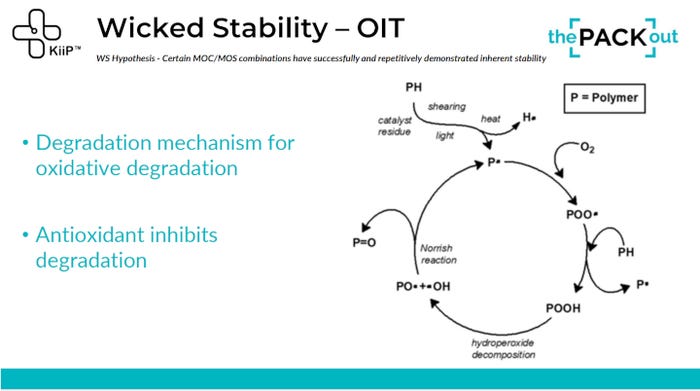
An abundance of data.
Market access authorities (MAAs), including the Food and Drug Administration (FDA), have been receptive to Wicked Stability’s hypothesis from the get-go — but a positive reception does not forego the need for hard evidence. Toward this end, the MAA workstream has been sifting through public databases for stability data to back up Wicked Stability’s theory.
Haley Schrauben, Product Manager, Oliver Healthcare Packaging, shared encouraging results: “What we found was very few records related to any sort of stability failure — and of those that were found, root cause was not back to material degradation. This was a huge piece of evidence that we want to take back to MAAs.”
Wendy Mach, Senior Sterilization Scientist for Integra LifeSciences, and Chris Sarantos, Principal of Shine Technologies, are currently sifting through the manufacturer and user facility device experience database (MAUDE) for evidence of stability failures. Of the approximately 1,500 FDA recalls perused so far, Mach reports that the group has identified only one that may have been related to shelf life.
Additional workstreams are reaching out to medical device manufacturers (MDMs) and sterile product manufacturers (SPMs) to submit their stability data for assessment against the Wicked Stability hypothesis. Information will be gathered in a blind repository, which will be collated by an independent data collector.
During thePACKout session, Schrauben appealed to attendees for help: “If you’re one of those companies that wants to participate, I encourage you; this is a call for action. Start pulling that data out of your validation reports if you don’t have it aggregated in a separate location.”
Progress is also being made in regard to leveraging data from the Medical Packaging Transition Project (MPTP) to release a DuPont authored summary report. DuPont is responsible for sending out no-objection agreements to the study’s participants (approximately 160 agreements are needed to do so) allowing DuPont to simplify the stability testing results that KiiP believes will support the Wicked Stability hypothesis. During the presentation, Jen Benolken, Medical Device & Regulatory Specialist, Package Engineering, DuPont Tyvek Medical Packaging, appealed to attendees for help in getting these letters to the right people.
Ultimately, the Wicked Stability team’s goal is to aggregate all of these data pools for analysis and authorship of a peer-reviewed publication that provides MAAs with evidence to support its claim. Benolken recently reported that the team is on the cusp of entering into discussions with MAAs to ensure they are gathering the right data to prove that materials commonly used in medical device packaging are stable.
Testing the hypothesis.
Wicked Stability is also making headway in putting its hypothesis to the test. Henk Blom, VP of Research and Technology, PAXXUS, updated attendees on the team’s preparations for measuring MOC antioxidant concentrations using OIT.
“We have begun to collect materials and design a rather large protocol using just low-density polyethylene; no multilayer structures,” he said. “We’re just starting simple: crawl before we walk before we run.”
Materials include two resins (one with antioxidant and one without), which have been converted into two rolls of film. Since thePACKout session, the film has been cut into samples and labeled. At present, the samples are being prepped for exposure to ethylene oxide and gamma sterilization, as well as various heat applications prior to being tested through OIT. Materials and the use of equipment for prepping and testing samples were donated free of charge.
“The support from industry has been incredible, because we’re going to need all sorts of help to do this,” said Blom.
As the team works toward engaging MAAs, Blom acknowledges that no promises have been made, and many questions remain unanswered. He encouraged attendees to believe in the science and keep challenging the hypothesis. And at the end of the day, the hope is that it remains valid.

To get involved, scan the QR code. Or reach out to the new Wicked Stability project lead, Jordan Montgomery, Distinguished Packaging Engineer at Medtronic, as Rod Patch has recently and voluntarily transitioned out of the project lead role.
About the Author(s)
You May Also Like



.png?width=300&auto=webp&quality=80&disable=upscale)
