How Working with a Molder with Multiple Capabilities Could Influence Design and Development
What can the diagnostics industry learn from the hotel amenities industry? Plenty.
July 14, 2017
|
Currier Plastics works to ensure blow molded bottles and injection molded closures "mate well together," said Ron Ringleben, vice president of business development. Image courtesy Currier Plastics. |
Currier Plastics, a custom plastics molder specializing in injection molding and blow molding (both extrusion and injection stretch), has been producing amenities bottles and closures for years. To meet hotels' custom design needs as well as their budgets, the 35-year-old company invested in a range of equipment and efficiencies to become a leading producer. One of its molding machines, for instance, produces 140 million bottles annually on one machine, Ron Ringleben, vice president of business development, told Qmed. The company just purchased its second electronic molding machine for better statistical process control, with one more to come.
"We design and produce all parts so they mate well together," he said, speaking of the company's ability to blow mold bottles and injection mold closures. "This allows for a faster development time, helping our customers' engineering development."
About five years ago Currier started producing components for the diagnostics industry and found that its amenities experience prepared the company well. Its injection and blow molding expertise--under one roof--have enabled the company to design and manufacture well-mated IVD components that minimize failures on production lines and in the field.
"For two components to come together, the features on both components have to match," Ringleben said. "We've invested in technologies to monitor wall thickness, vision inspection, as well as top-load pressure and other attributes."
For instance, "customers cannot have bottles that leak," added Elizabeth Roberts, marketing manager. "We know how to properly seal a container and make sure the product works as designed."
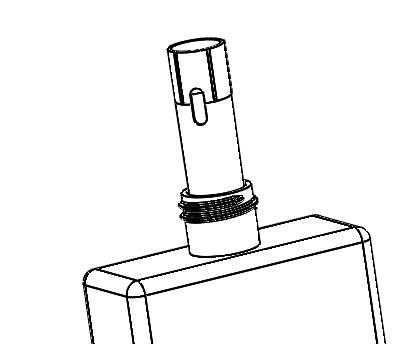
|
Image of an injection molded tube inserted into a blow molded container courtesy Currier Plastics. |
In vitro diagnostics manufacturers' needs for their reagents and assays are surprisingly similar to those of hotels. Volume is one. "Some IVD clients require from a few million to nearly a billion components a year," said Ringleben. Quality is key, too. "With IVDs, you need to achieve a specific tolerance range so that the components can fit into the fill line equipment and the diagnostic test instrument."
Consequently, "we've been forced to be very efficient," Ringleben continued. "We've added capacity to produce products faster with higher quality output." Currier maintains a zero-defect quality policy, aided by in-line check systems using vision systems, Roberts added.
Currier's last IVD project entailed developing the plastic consumables for reagents and assays at the same time as the customer developed its testing instrument. Such early involvement in development is critical. "Shape, angle, and optical clarity are all important and can affect the way reagents sit in the containers," he said. The containers also need to be designed so they can be handled efficiently on filling lines. "We can help designers with 3-D CAD and 3-D printed prototypes before moving to production," he added. "We can double check quality before we cut steel for tools. These quality steps are taken to make sure that all components are manufactured to specifications."
Materials selection is another issue to be addressed early, and Currier can help. "We are able to recommend the best products and materials that work together," said Roberts. "Materials can interact with reagents. We can offer and evaluate FDA-grade resins for compatibility testing."
Added Ringleben: "We'll start with user needs and turn those into design requirements." For instance, "some containers need to be able to hold reagents for two years without reacting. This is the iterative process that allows for many design options to be evaluated."
|
Currier Plastics's "clean" environments. Image courtesy Currier Plastics. |
Given Currier's recent entry into healthcare, the company is scaling up to meet the industry's stringent particulate controls. It currently operates several of its machines in "clean" environments and offers cleanroom assembly. "We take an affordable, portable approach with 'cleanroom cells' that can be moved around to meet different component needs," he said. "We add covers and filters to molding machines as needed, covering and filtering the areas of the machines that are exposed to the product path, and we wheel up portable tents for the downstream processing. We control products in their own 'frame' before they move into the cleanroom assembly environments." The company is also considering further investments in cleanroom manufacturing/assembly, depending upon customer need.
Currier can also help with education. While most large-sized medical device companies have a "general understanding" of the different molding technologies, Ringleben said, he still sees a need for education, especially for small to medium-sized companies, so Currier offers courses on the different processes. "Better decisions could be made about design," he said. "It also allows companies to accelerate development."
Working with a molder with multiple capabilities also allows companies to work with fewer suppliers, said Ringleben. "Companies have fewer resources than they did five years ago, and they need to consolidate," he said. "Companies are now asking us to evaluate several products. They also like our nimble response and accelerated project completion.
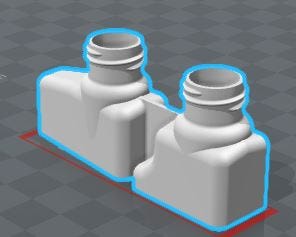
|
Image of containers with dual chamber necks courtesy Currier Plastics. |
"Some companies struggle with finding the right partner with plastics expertise," he continued. "Many of our employees have four-year plastics degrees," added Roberts. "But we still see the need for developing local talent, so we helped develop a plastics program at Cayuga Community College. One of our quality engineers is the course instructor, and we donated a machine and helped set up the lab." The first course was held in July 2016.
Currier Plastics will be exhibiting at the upcoming AACC meeting July 30 to August 3, 2017, in San Diego as well as Medical Design & Manufacturing East 2018 June 12-18, 2018 in New York City.
Don't miss the upcoming MD&M Minneapolis Conference and Expo, November 8-9, 2017.
Daphne Allen is executive editor of Pharmaceutical & Medical Packaging News and a contributor to MD&DI and Qmed. Reach her at [email protected] and on Twitter at @daphneallen
About the Author(s)
You May Also Like





.png?width=300&auto=webp&quality=80&disable=upscale)
