Digitized DHRs and Flexible Manufacturing in the Age of COVID-19
Paperless device history records could streamline operations and readily capture data, easing access anytime, anywhere.
July 8, 2020
The COVID-19 outbreak has forced rapid adaptation across the entire healthcare industry. With a dire need for medical devices and personal protective equipment (PPE), manufacturers have quickly sought to retool factories and repurpose capacity to help address the health crisis created by the novel coronavirus. While manufacturers of N95 masks, ventilators, and other equipment have ramped up production, makers of all kinds—from vodka and hockey equipment to vacuum cleaners and hand dryers—have also pivoted to produce products like hand sanitizer, medical shields, and ventilators in response to shortages created by the pandemic.
Even as manufacturers adapt with extraordinary agility to fulfill skyrocketing demand, it’s unclear how quickly units can consistently reach those who need them. Manufacturers must be able to balance speed with compliance, yet as production expands to meet immediate needs, paper-based processes can slow production down or cast doubt on the quality of the lifesaving equipment.
Manufacturing must be nimble and scalable to meet the demands of changing environments, and during times of massive health crises, scaling and accelerating production is especially critical.
Digitized vs. Paper-Based Device History Records: Adapting and Accelerating Production
To help companies fight the pandemic, Medtronic shared its PB 560 portable ventilator design specifications and manufacturing procedures for free, allowing companies to access the design and manufacture the ventilator hardware. Yet, for a manufacturer pivoting from producing core products to scaling up production of a ventilator, how agile is its production environment?
For medical device and diagnostics manufacturers investing in factory automation such as manufacturing execution systems (MES), their efforts are too often impeded by critical processes that remain paper based, such as device history records (DHRs). Many companies live with these rigid, paper-based processes on the shop floor because they believe it takes too long and is too costly to implement a new, digital production system and adjust to new processes. In a recent MasterControl poll, 66.7% of life sciences professionals cited project length as the No. 1 reason they haven’t digitized their production record process yet.
The hesitation is understandable. Depending on complexity, an MES system can take months or even years to implement. According to iBASEt, custom-built MES configurations based on MES tool kits can run a license-to-service-dollars ratio upwards of 1:5, meaning that for every $10,000 spent on licenses, manufacturers may actually spend $50,000 in services. A commercial off-the-shelf MES solution can be in the range of 1:2, according to the MES provider.
A configurable, cloud-based digital production system with electronic DHR (eDHR) provides an even better ratio of 3:1 and can be implemented within eight weeks, from planning/discovery through building/testing/training to go-live. Once implemented, the system can deliver results fast.
For manufacturers using a paper-based or partially digital system to manage their DHRs, building and maintaining complete, orderly records per U.S. FDA 21 CFR Part 820 requirements is an inherently inefficient, often error-prone, process. Relying on slow, manual DHR processes often leads to finished product sitting unshipped until the records are reviewed and approved.
An eDHR system gives manufacturers the ability to accelerate production and maintain agility in changing environments, without the common pains involved with pushing paper.
While paper-based DHR processes are inherently slow, cumbersome, and prone to errors, eDHRs can streamline the process by eliminating data entry errors, validating in real-time and enforcing quality controls without slowing production.
While companies that rely on manual, paper-based DHR systems are limited in their ability to track manufacturing changes, eDHRs can be integrated with a quality management system (QMS) to provide real-time nonconformance- and deviation-tracking tools to identify quality events and take corrective action in-line.
And while paper DHRs don’t synchronize across a company’s multiple enterprise systems, severely limiting the throughput of data between critical business functions, eDHRs ensure right-first-time data collection and enable that data to be captured and stored in a format that can be readily accessed, analyzed, and connected—enabling the free flow of data and communication between different systems and people.
Digitizing paper DHRs leads to truly paperless manufacturing, technology-driven process improvements and integration of systems and processes. Ultimately, that means a faster, more flexible manufacturing environment.
Speed to Build Device History Records for New Medical Devices
It can take weeks to build from scratch a master DHR template for a new medical device. And it can take weeks to review, sign off and close out a DHR using paper.
We recently set out to see how digitizing DHRs could affect operations in a time of COVID-19-driven change, using our Manufacturing Excellence solution.
“Building one new, paper-based DHR typically takes one full-time equivalent between two days and two weeks, or even longer, depending on complexity,” according to MasterControl SVP of Strategic Growth Brian Curran. “Medtronic’s DHRs fall on the less complicated side, so we estimated two to three days for each paper record.”
The team was able to take Medtronic’s open-source design and build an eDHR in approximately two hours. It required 60 minutes to review and add Medtronic’s 24 open-sourced design and documents, and then 45 minutes to build and create two executable DHR templates.
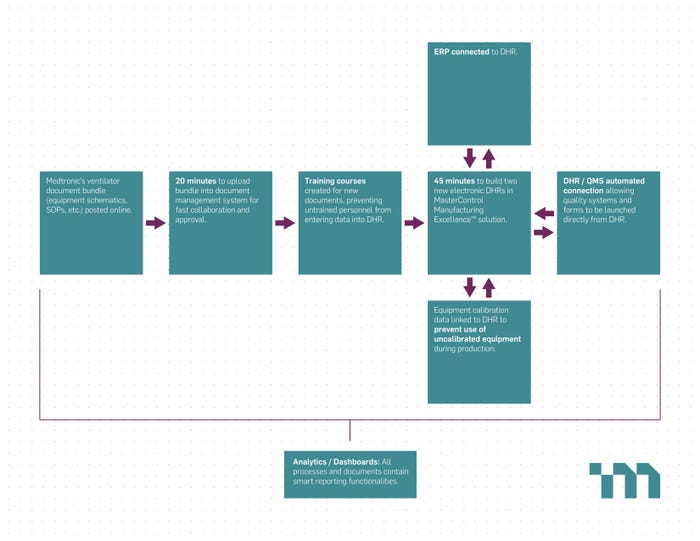
Flowchart showing workflow/process and time savings courtesy of MasterControl.
The configurable, digitized DHRs were designed to have the familiar look of paper records, meaning they resemble the records used by most manufacturers today. When configuring and assembling the DHR output, predecessors and conditional paths can be set up at each level. Tables and repeatable operations can support a process where multiple iterations of a step are required, such as quality control (QC) inspections or viable counts that are repeated on a continuous time schedule. Templates make building subsequent master templates faster and easier, even for product variations.
“Paper takes weeks. With digitization, we were able to take an existing design and create two DHRs in 45 minutes,” according to Curran. “Digitization lets medical device manufacturers easily create and manage their DHRs and quickly ship their lifesaving products.”
Speed and flexibility are critical when adapting to a rapid shift in demand, but in highly regulated industries like medical devices, changing over and scaling up a new product line requires tight alignment with product development, quality departments, resource planning, and supply chains.
Integrating eDHRs With a Platform Approach
To achieve the flexibility and speed associated with digitizing production and adopting eDHRs, there must be a more holistic view of data across the entire product life cycle, from development through manufacturing and quality. Taking a platform approach to digitally managing design history files (DHFs), technical documents, and DHRs smooths the design transfer to production and can accelerate critical exchanges of data, processes, and knowledge throughout the organization.
For instance, integrating eDHRs with standard operating procedures (SOPs) in your QMS, bill of materials (BOM) in your enterprise resource planning (ERP) system, and operator training or work instructions in your learning management system (LMS) mean that in a short period of time, a manufacturer can efficiently track—and maintain in one place—much of the information needed for the device being produced, including design, components, orders, machine data, and operator training.
The tighter the integration with core systems, the greater the impact. For instance, deploying an eDHR application that has native connectivity with your QMS means you can ensure operators always use the correct version of a work instruction. It also means operators have the ability to launch deviations directly from the DHR to ensure in-line quality assurance. Automatically storing eDHRs in a QMS eliminates manually routing of paper records from the shop floor to the quality department. And with review-by-exception functionality, an eDHR solution can identify for quality which processes fell outside of the acceptable limits without passing paper back and forth between people. In the COVID-19 era, such witness verifications and signoffs on a digital device essentially work as social-distanced DHR review and approval.
By digitally integrating eDHRs and capitalizing on platform dynamics, medical device manufacturers are able to build quality into their manufacturing system, improve right-first-time (RFT) metrics, and increase shop floor productivity. The result is that manufacturers can ship products faster without undermining product quality.
Conclusion
As medical technology manufacturers re-evaluate and adjust their processes to help address the global health crisis—and to prepare for the new normal that comes next—speed and flexibility will continue to define modern manufacturing. The COVID-19 pandemic has brought to light the importance of digitization in flexible manufacturing. In manufacturing environments where peripheral, yet critical, processes continue to rely on slow, manual paper-based systems, digitizing DHRs can fill that gap.
About the Author(s)
You May Also Like




